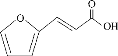
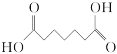
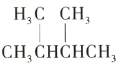��Ŀ����
����Ŀ�����й��ڷ�ӦN2(g)��3H2(g) ![]() 2NH3(g)����H<0��ͼʾ���Ӧ����������ϵ���(����)
2NH3(g)����H<0��ͼʾ���Ӧ����������ϵ���(����)
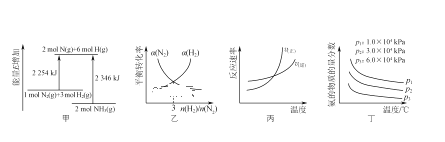
A.��ͼ��֪N2(g)��3H2(g) ![]() 2NH3(g)����H����92 kJ��mol��1
2NH3(g)����H����92 kJ��mol��1
B.ͼ�ұ�ʾ�ﵽƽ��ʱN2��H2��ת����(��)��n(H2)/n(N2)��ֵ�ı仯
C.ͼ����ʾ�����淴Ӧ�������¶ȵı仯
D.ͼ����ʾ��Ӧ��ƽ��ʱ����������а������ʵ����������¶ȡ�ѹǿ�ı仯
���𰸡�A
��������
A.��ͼ��֪��Ӧ�������������������������������Է�Ӧ�Ƿ��ȷ�Ӧ��1mol������3mol������ȫ��Ӧ�ų�����Ϊ��2346-2254=92kJ�������ȷ�Ӧ����ʽΪ��N2��g��+3H2��g��![]() 2NH3��g������H=-92kJmol-1����A��ȷ��
2NH3��g������H=-92kJmol-1����A��ȷ��
B.���������ʵ���Խ������ת����Խ������������ת����ԽС����B����
C.�����¶������淴Ӧ���ʶ�����ƽ�������ƶ����������߽����Ӧ�淴Ӧ���ʴ�������Ӧ�����ʣ���C����
D.ѹǿԽ��������������Խ������ԽС����D����
�ʴ�ѡA��

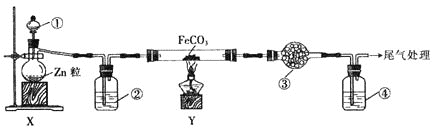
����Ŀ��ʵ������ijЩ�������ȡ���ռ���β������װ����ͼ��ʾ��ʡ�Լгֺ;���װ�ã������ô�װ�úͱ����ṩ������������ʵ�飬�������ѡ����

ѡ�� | a�е����� | b�е����� | c���ռ������� | d�е����� |
A | Ũ��ˮ | CaO | NH3 | H2O |
B | Ũ���� | Na2SO3 | SO2 | NaOH��Һ |
C | ϡ���� | Cu | NO2 | H2O |
D | Ũ���� | MnO2 | Cl2 | NaOH��Һ |