��Ŀ����
����Ŀ����Ҫ�������пո�����ա�
��1��һ�������Ҵ��뱽�Ļ�����������Ľ����Ʒ�Ӧ��������11.2L�������ڱ�״���£������˻����ȼ��������108gˮ��
���������������__________________
��2��ij����A�������ۺϳɱ���µ��ܶ���3.214g/L����֪������̼��������Ϊ5��1����
�ٸ�������Է���������_________,
�����������һ��ȡ������4�֣�д�������Ľṹ��ʽ��___________________________________________
��3��5.8g�л�����ȫȼ�գ�ֻ����CO2��H2O�������������Ϊ1:1(ͬѹͬ��)����������ͨ����ʯ�ң���ʯ����������18.6g��ͬ�����л�����0.1mol������ȫ����������Ӧ����֪���л���Կ���������ܶ�Ϊ2����ע�⣺�ǻ�����ֱ������˫���ϣ�
���л���ķ���ʽ___________________________________________________________
���л���Ľṹ��ʽ___________________________________________________________
���𰸡�78g 72 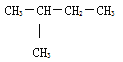 C3H6O CH2=CHCH2OH
C3H6O CH2=CHCH2OH
��������
��1�������11.2L���������ʵ���Ϊ��![]() =0.5mol���������к���C2H5OH�����ʵ���Ϊ��0.5mol��2=1mol��1mol�Ҵ���ȫȼ�ջ�����ˮ�����ʵ���Ϊ��
=0.5mol���������к���C2H5OH�����ʵ���Ϊ��0.5mol��2=1mol��1mol�Ҵ���ȫȼ�ջ�����ˮ�����ʵ���Ϊ��![]() =3mol������Ϊ��18g/mol��3mol=54g�������ȼ��������108gˮ���������б���ȫȼ������ˮ������Ϊ��108g-54g=54g��ˮ�����ʵ���Ϊ��
=3mol������Ϊ��18g/mol��3mol=54g�������ȼ��������108gˮ���������б���ȫȼ������ˮ������Ϊ��108g-54g=54g��ˮ�����ʵ���Ϊ��![]() =3mol��3molˮ�к���6molHԭ�ӣ������ʵ���Ϊ��
=3mol��3molˮ�к���6molHԭ�ӣ������ʵ���Ϊ��![]() =1mol�����ԣ�������к���C2H5OH�����ʵ���Ϊ1mol��������б�������Ϊ��78g/mol��1mol=78g���ʴ�Ϊ��78g��
=1mol�����ԣ�������к���C2H5OH�����ʵ���Ϊ1mol��������б�������Ϊ��78g/mol��1mol=78g���ʴ�Ϊ��78g��
��2���ٸ�����̼��������Ϊ5��1��C��HԪ�ص����ʵ���֮��Ϊ��![]() ��
��![]() =5��12�����������ʽΪ��C5H12��������Ħ������Ϊ��M=��Vm=3.214g/L��22.4L/mol=72g/mol������������Է�������Ϊ72���ʴ�Ϊ��72��
=5��12�����������ʽΪ��C5H12��������Ħ������Ϊ��M=��Vm=3.214g/L��22.4L/mol=72g/mol������������Է�������Ϊ72���ʴ�Ϊ��72��
�ڸ�������Է�������Ϊ72�����Ը��л���ķ���ʽΪ��C5H12��C5H12Ϊ���飬�����ͬ���칹���Ϊ�����顢������������飬�����������һ�ȴ�����3�֡��������һ�ȴ����ͬ���칹����4�֡��������һ�ȴ����ͬ���칹����1�֣�������������������ͬ���칹��Ϊ��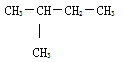 ���ʴ�Ϊ��
���ʴ�Ϊ��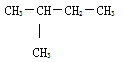 ��
��
��3���ٸ��л���Կ���������ܶ�Ϊ2�����л�����Է�������=29��2=58��5.8g�л�������ʵ���Ϊ![]() =0.1mol������ȼ������CO2��H2O�����������Ϊ1��1����ʯ����������18.6gΪCO2��H2O����������n(CO2)=n(H2O)=
=0.1mol������ȼ������CO2��H2O�����������Ϊ1��1����ʯ����������18.6gΪCO2��H2O����������n(CO2)=n(H2O)=![]() =0.3mol�����л��������N(C)=
=0.3mol������������N(C)=![]() =3��N(H)=
=3��N(H)=![]() =6��N(O)=
=6��N(O)=![]() =1�����л������ʽΪ��C3H6O���ʴ�Ϊ��C3H6O��
=1�����л������ʽΪ��C3H6O���ʴ�Ϊ��C3H6O��
��ͬ�����л�����0.1mol������ȫ����������Ӧ�����л�����Ӻ���1��-OH���л������ʽΪC3H6O������1��C=C˫��������ṹ��ʽΪCH2=CHCH2OH���ʴ�Ϊ��CH2=CHCH2OH��

����Ŀ����֪��ѧ��Ӧ��:Fe(s)+CO2(g)![]() FeO(s)+CO(g),�仯ѧƽ�ⳣ��ΪK1;��ѧ��Ӧ��:Fe(s)+H2O(g)
FeO(s)+CO(g),�仯ѧƽ�ⳣ��ΪK1;��ѧ��Ӧ��:Fe(s)+H2O(g)![]() FeO(s)+H2(g),�仯ѧƽ�ⳣ��ΪK2,���¶�973 K��1173 K�������,K1��K2��ֵ�ֱ�����:
FeO(s)+H2(g),�仯ѧƽ�ⳣ��ΪK2,���¶�973 K��1173 K�������,K1��K2��ֵ�ֱ�����:
�¶� | K1 | K2 |
973 K | 1.47 | 2.38 |
1 173 K | 2.15 | 1.67 |
(1)ͨ�������е���ֵ�����ƶ�:��Ӧ����_______(��������������������)��Ӧ��
(2)���з�Ӧ��:CO2(g)+H2(g)![]() CO(g)+H2O(g),����д���÷�Ӧ��ƽ�ⳣ��K3�ı���ʽ:K3=______��
CO(g)+H2O(g),����д���÷�Ӧ��ƽ�ⳣ��K3�ı���ʽ:K3=______��
(3)���ݷ�Ӧ����ڿ��Ƶ���K1��K2��K3֮��Ĺ�ϵʽΪ__________,�ݴ˹�ϵʽ���ϱ�����,���ƶϳ���Ӧ����________(��������������������)��Ӧ��
(4)Ҫʹ��Ӧ����һ�������½�����ƽ��������Ӧ�����ƶ�,�ɲ�ȡ�Ĵ�ʩ��______ ��_____ (��д��ĸ���)��
A.��С��Ӧ�������ݻ� B.����Ӧ�������ݻ�
C.�����¶� D.ʹ�ú��ʵĴ���
E.�跨��Сƽ����ϵ�е�CO��Ũ��
(5)ͼ�ס��ҷֱ��ʾ��Ӧ����t1ʱ�̴ﵽƽ��,��t2ʱ����ı�ij�������������仯�����:
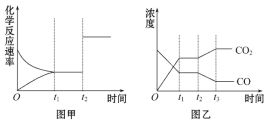
��ͼ����t2ʱ�̷����ı��������__________��
��ͼ����t2ʱ�̷����ı��������__________��