��Ŀ����
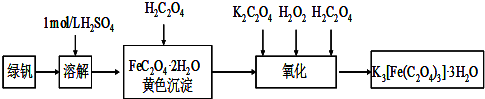
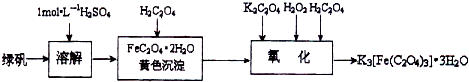
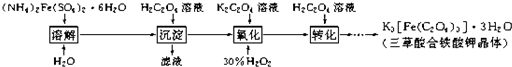
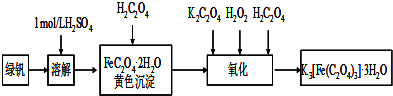
 �����������ؾ��壨K3[Fe��C2O4��3]?xH2O����һ�ֹ������ϣ���������Ӱ����ɫӡˢ��Ϊ�ⶨ�þ��������ĺ����ͽᾧˮ�ĺ�����ijʵ��С����������ʵ�飺
�����������ؾ��壨K3[Fe��C2O4��3]?xH2O����һ�ֹ������ϣ���������Ӱ����ɫӡˢ��Ϊ�ⶨ�þ��������ĺ����ͽᾧˮ�ĺ�����ijʵ��С����������ʵ�飺
��1�������ϵøþ�����110�����ȫʧȥ�ᾧˮ�����ǽ�����ϴ������������أ���¼�������������м�����ϸ�������������ؾ��壬��������¼������������110��һ��ʱ�䣬���ڿ�������ȴ����������¼����������ᾧˮ�����������ʵ������е���������______��______��
��2����֪2KMnO4+5H2C2O4+3H2SO4��K2SO4+10CO2��+2MnSO4+8H2O���ֳ���5.000g�����������ؾ��壬���Ƴ�250ml��Һ��ȡ������Һ25.00ml����ƿ�У���ϡH2SO4�ữ���μ�KMnO4��Һ�������ǡ��ȫ���������ɶ�����̼����Ӧ�����Һ�м���һС��п�ۣ�������dz��ɫ�պ���ʧ�����ˣ�ϴ�ӣ������˼�ϴ��������Һ�ռ�����ƿ�У���ʱ����Һ�Գ����ԣ�����п�۵�Ŀ����______��
��3����0.010mol/L KMnO4��Һ�ζ���һ����������Һ���յ㣬����KMnO4��Һ20.02ml���ζ���MnO4-����ԭ��Mn2+��д��������Ӧ�����ӷ���ʽ______��
��4������ʵ��ζ�ʱ��ָʾ��Ӧ��______����ӡ����ӡ���������жϵζ��յ㣺______��
��5���ڣ�2���ⲽ���У��������KMnO4����Һ�������㣬�ɵ��²�õ�������______����ѡ�ƫ�͡���ƫ�ߡ������䡱��
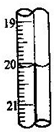
��6���ظ���2����3������������ζ�����0.010mol/L KMnO4��Һ19.98ml����ʵ���øþ�����������������Ϊ______������ɫ�ֱ��ζ��ܵζ����������ͼ��ʾ�������¼�¼��������ȷ����______
A��20.00mL����B.20.0mL���� C.20.10mL���� D.20.1mL��
�⣺��1��ʵ������е����������Ⱥ�û���ڸ���������ȴ��������Ⱥ�û�н��к��ز�����
�ʴ�Ϊ�����Ⱥ�û���ڸ���������ȴ��������Ⱥ�û�н��к��ز�����
��2���ɣ�3��������֪�����ø�����زⶨ�������ӣ��ʼ���п�۵�Ŀ���ǽ�Fe3+ǡ�û�ԭ��Fe2+��Ϊ��һ���ⶨ��Ԫ�صĺ���������
�ʴ�Ϊ����Fe3+��ԭ��Fe2+��Ϊ��һ���ⶨ��Ԫ�صĺ���������
��3�����������������Һ�о���ǿ��������������������Ϊ�����ӣ���������ԭΪ�����ӣ����������ӷ�ӦΪ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O��
�ʴ�Ϊ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O��
��4�����������ҺΪ�Ϻ�ɫ������������Һ��ɫ�仯��ָʾ��Ӧ���յ㣬����Ҫָʾ�������������Һ����ɫ���������ӷ�Ӧ��ǡ�÷�Ӧ��������һ�θ��������Һ��ɫ����Ӳ���ɫ֤����Ӧ�յ㣻
�ʴ�Ϊ�����ӣ�������Һ�г����Ϻ�ɫ�����������ڲ�����ɫ���ͱ��������յ㣻
��5�������KMnO4����Һ�������㣬���²������ʣ�࣬�ٵζ���������ʱ�������������ط�Ӧ��ʹ�ĵζ������������ĵĸ�����ص����ƫ���²ⶨ���������ӵ����ʵ���ƫ����Ԫ�ص�����ƫ����ⶨ����Ԫ�ص���������ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��6�����ظ���2������3�����裬�ζ�����0.01moL/KMnO4��Һƽ�����Ϊ19.98mL��
5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O
5mol 1mol
n��Fe2+�� 0.010��0.01998mol
���Ƴ�250moL��Һ��ȡ������Һ25.00moL���е��к͵ζ�ʵ�飬�������������ʵ���n��Fe2+��=5��0.010��0.01998mol������250ml��Һ�������������ʵ���Ϊ��10��5��0.010��0.01998mol���������������أ�Fe��=��10��5��0.010��0.01998mol��56 g/mol����5.000 g��100%=11.12%��
��ͼ��֪���Ҳ�Ķ��������С0.01�����Ҳ�ζ��Ķ���Ϊ20.00mL����ѡA��
�ʴ�Ϊ��11.12%��A��
��������1�����Ⱥ�û���ڸ���������ȴ��������Ⱥ�û�н��к��ز�����
��2���ɣ�3��������֪�����ø�����زⶨ�������ӣ��ʼ���п�۵�Ŀ���ǽ�Fe3+ǡ�û�ԭ��Fe2+��Ϊ��һ���ⶨ��Ԫ�صĺ���������
��3�����������������������������·�Ӧ���������ӡ���������ˮ����ƽ��д����ʽ��
��4��KMnO4��Һ����Ϊ�Ϻ�ɫ��������Һ�г����Ϻ�ɫ�����������ڲ�����ɫ���ͱ��������յ㣻
��5�������KMnO4����Һ�������㣬���²������ʣ�࣬�ٵζ���������ʱ�������������ط�Ӧ��ʹ�ĵζ������������ĵĸ�����ص����ƫ��
��6���������ӷ���ʽ����n��Fe2+��������Ԫ���غ㣬����m=nM���㾧������Ԫ�ص��������ٸ�����������������㣻��ͼ��֪���Ҳ�Ķ��������С0.01�����Ҳ�ζ��Ķ���Ϊ20.00mL��
���������⿼���Ϊ�ۺϣ��漰����Һ�����ơ��ζ�������ָʾ��ѡ��������ԭ��Ӧ�����ӷ���ʽ����д����ѧ����ʽ�ļ��㣬ע�����ʵ��֪ʶ�Ļ��ۣ�����ʵ�鲽�衢ԭ����ע����������⣮
�ʴ�Ϊ�����Ⱥ�û���ڸ���������ȴ��������Ⱥ�û�н��к��ز�����
��2���ɣ�3��������֪�����ø�����زⶨ�������ӣ��ʼ���п�۵�Ŀ���ǽ�Fe3+ǡ�û�ԭ��Fe2+��Ϊ��һ���ⶨ��Ԫ�صĺ���������
�ʴ�Ϊ����Fe3+��ԭ��Fe2+��Ϊ��һ���ⶨ��Ԫ�صĺ���������
��3�����������������Һ�о���ǿ��������������������Ϊ�����ӣ���������ԭΪ�����ӣ����������ӷ�ӦΪ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O��
�ʴ�Ϊ��5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O��
��4�����������ҺΪ�Ϻ�ɫ������������Һ��ɫ�仯��ָʾ��Ӧ���յ㣬����Ҫָʾ�������������Һ����ɫ���������ӷ�Ӧ��ǡ�÷�Ӧ��������һ�θ��������Һ��ɫ����Ӳ���ɫ֤����Ӧ�յ㣻
�ʴ�Ϊ�����ӣ�������Һ�г����Ϻ�ɫ�����������ڲ�����ɫ���ͱ��������յ㣻
��5�������KMnO4����Һ�������㣬���²������ʣ�࣬�ٵζ���������ʱ�������������ط�Ӧ��ʹ�ĵζ������������ĵĸ�����ص����ƫ���²ⶨ���������ӵ����ʵ���ƫ����Ԫ�ص�����ƫ����ⶨ����Ԫ�ص���������ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��6�����ظ���2������3�����裬�ζ�����0.01moL/KMnO4��Һƽ�����Ϊ19.98mL��
5Fe2++MnO4-+8H+=5Fe3++Mn2++4H2O
5mol 1mol
n��Fe2+�� 0.010��0.01998mol
���Ƴ�250moL��Һ��ȡ������Һ25.00moL���е��к͵ζ�ʵ�飬�������������ʵ���n��Fe2+��=5��0.010��0.01998mol������250ml��Һ�������������ʵ���Ϊ��10��5��0.010��0.01998mol���������������أ�Fe��=��10��5��0.010��0.01998mol��56 g/mol����5.000 g��100%=11.12%��
��ͼ��֪���Ҳ�Ķ��������С0.01�����Ҳ�ζ��Ķ���Ϊ20.00mL����ѡA��
�ʴ�Ϊ��11.12%��A��
��������1�����Ⱥ�û���ڸ���������ȴ��������Ⱥ�û�н��к��ز�����
��2���ɣ�3��������֪�����ø�����زⶨ�������ӣ��ʼ���п�۵�Ŀ���ǽ�Fe3+ǡ�û�ԭ��Fe2+��Ϊ��һ���ⶨ��Ԫ�صĺ���������
��3�����������������������������·�Ӧ���������ӡ���������ˮ����ƽ��д����ʽ��
��4��KMnO4��Һ����Ϊ�Ϻ�ɫ��������Һ�г����Ϻ�ɫ�����������ڲ�����ɫ���ͱ��������յ㣻
��5�������KMnO4����Һ�������㣬���²������ʣ�࣬�ٵζ���������ʱ�������������ط�Ӧ��ʹ�ĵζ������������ĵĸ�����ص����ƫ��
��6���������ӷ���ʽ����n��Fe2+��������Ԫ���غ㣬����m=nM���㾧������Ԫ�ص��������ٸ�����������������㣻��ͼ��֪���Ҳ�Ķ��������С0.01�����Ҳ�ζ��Ķ���Ϊ20.00mL��
���������⿼���Ϊ�ۺϣ��漰����Һ�����ơ��ζ�������ָʾ��ѡ��������ԭ��Ӧ�����ӷ���ʽ����д����ѧ����ʽ�ļ��㣬ע�����ʵ��֪ʶ�Ļ��ۣ�����ʵ�鲽�衢ԭ����ע����������⣮

��ϰ��ϵ�д�
 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д� ���������ν�ϵ�д�
���������ν�ϵ�д�
�����Ŀ