��Ŀ����
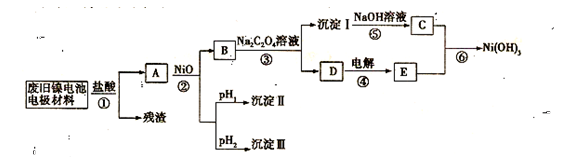
����Ч�ļ���ȼ�ϵ�ز��ò�Ϊ�缫���ϣ����缫�Ϸֱ�ͨ��CH4��O2�������ΪKOH��Һ��ij�о�С�齫��������ȼ�ϵ�ش�������Ϊ��Դ�����б����Ȼ�����Һ(���м��η�̪)���ʵ�飬��ͼ��ʾ��
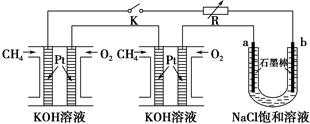
�ش��������⣺
(1)����ȼ�ϵ�������������ĵ缫��Ӧ�ֱ�Ϊ________________________ ��________________________��
(2)�պ�K���غ�a��b�缫�Ͼ����������������a�缫�ϵõ�����________����������________��(�a����b��)��������ֺ�ɫ������Ȼ�����Һ���ܻ�ѧ����ʽΪ________________________����a��b�����IJ������Ӧ�ɵõ���84������Һ����Ч�ɷ�NaClO����������¿��á�84������Һ����SO2����Ӧ�����ӷ���ʽΪ________________________��
(3)��ÿ����ؼ���ͨ����Ϊ1 L(��״��)���ҷ�Ӧ��ȫ��������������ܲ������������Ϊ________L(��״��)��

�ش��������⣺
(1)����ȼ�ϵ�������������ĵ缫��Ӧ�ֱ�Ϊ________________________ ��________________________��
(2)�պ�K���غ�a��b�缫�Ͼ����������������a�缫�ϵõ�����________����������________��(�a����b��)��������ֺ�ɫ������Ȼ�����Һ���ܻ�ѧ����ʽΪ________________________����a��b�����IJ������Ӧ�ɵõ���84������Һ����Ч�ɷ�NaClO����������¿��á�84������Һ����SO2����Ӧ�����ӷ���ʽΪ________________________��
(3)��ÿ����ؼ���ͨ����Ϊ1 L(��״��)���ҷ�Ӧ��ȫ��������������ܲ������������Ϊ________L(��״��)��
(1)O2��2H2O��4e��=4OH�� CH4��10OH����8e��=CO32����7H2O
(2)Cl2��b��2NaCl��2H2O 2NaOH��H2����Cl2����ClO����SO2��H2O=Cl����SO42����2H��
2NaOH��H2����Cl2����ClO����SO2��H2O=Cl����SO42����2H��
(3)4
(2)Cl2��b��2NaCl��2H2O
 2NaOH��H2����Cl2����ClO����SO2��H2O=Cl����SO42����2H��
2NaOH��H2����Cl2����ClO����SO2��H2O=Cl����SO42����2H��(3)4
(3)����CH4��10OH����8e��=CO32����7H2O��2Cl����2e��=Cl2���͵����غ�֪�������ϵʽΪCH4��8e����4Cl2�����Ա�״�����������Ϊ4 L��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
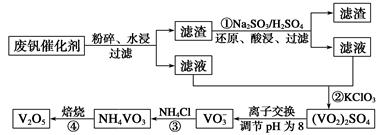
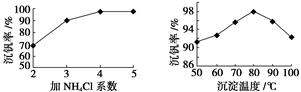
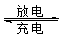 VO2+��H2O��V3+����س��ʱ�����ĵ缫��ӦʽΪ ��
VO2+��H2O��V3+����س��ʱ�����ĵ缫��ӦʽΪ ��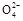 ֻ��PbO2���ƶ�
ֻ��PbO2���ƶ� 2PbSO4+2H2O������˵����ȷ����
2PbSO4+2H2O������˵����ȷ���� ������Ҫ����Դ���ʣ��о��״�������Ҫ���塣
������Ҫ����Դ���ʣ��о��״�������Ҫ���塣 ����ȡ�״����䷴ӦΪ��
����ȡ�״����䷴ӦΪ��

 ��
��  ���壬�ڸ��������ܴ���
���壬�ڸ��������ܴ��� ���ӡ���ع���ʱ������ӦΪ ��
���ӡ���ع���ʱ������ӦΪ ��
 ������
������ ��Ȼ����
��Ȼ����
 ���ù����б�������Ԫ���� ����������״����2.24L
���ù����б�������Ԫ���� ����������״����2.24L M+Ni(OH)2��MΪ����Ͻ�MHΪ��������ԭ�ӵĴ���Ͻ𡣵������ҺΪŨKOH��Һ��
M+Ni(OH)2��MΪ����Ͻ�MHΪ��������ԭ�ӵĴ���Ͻ𡣵������ҺΪŨKOH��Һ��