��Ŀ����
����Ŀ���ֽ�0.40 mol A�����0.20 mol B�������10 L���ܱ������У���һ��������ʹ�䷢����Ӧ��������C�������ʵ����ı仯��ͼ��
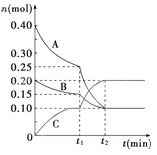
��1����t1��10 min����0��t1ʱ����C���ʵ�ƽ����Ӧ����Ϊ________________����Ӧ��t2ʱ�ﵽƽ�⣬�仯ѧ����ʽΪ_____________________________________��
��2���÷�Ӧt2ʱ�̷�Ӧ��ѧƽ�ⳣ��ΪK=________________________��
��3����ͼ�����߱仯���������t1ʱ�̸ı�ķ�Ӧ����������________��
A�������˴��� B�������˷�Ӧ�¶�
C�������������C D�������������
���𰸡� 1.0��10��3 mol��L��1��min��1 3A(g)��B(g)![]() 2C(g) 4��104 AD
2C(g) 4��104 AD
�������������������1������![]() ���㷴Ӧ���ʣ�����ϵ���ȵ������ʵ����仯���㷽��ʽ��ϵ������2�����ݷ�Ӧ����ʽ���㻯ѧƽ�ⳣ������3������ͼ��t1ʱ�̲�һ����ƽ��״̬��t1��t2��һ����ƽ���ƶ��������ʼӿ죻
���㷴Ӧ���ʣ�����ϵ���ȵ������ʵ����仯���㷽��ʽ��ϵ������2�����ݷ�Ӧ����ʽ���㻯ѧƽ�ⳣ������3������ͼ��t1ʱ�̲�һ����ƽ��״̬��t1��t2��һ����ƽ���ƶ��������ʼӿ죻
��������1������![]() ��0��t1ʱ����C���ʵ�ƽ����Ӧ����Ϊ
��0��t1ʱ����C���ʵ�ƽ����Ӧ����Ϊ![]() 1.0��10��3 mol��L��1��min��1 ��A��B��C���ʵ����ı仯�ֱ���0.3mol��0.1mol��0.2mol,
1.0��10��3 mol��L��1��min��1 ��A��B��C���ʵ����ı仯�ֱ���0.3mol��0.1mol��0.2mol,
����ϵ���ȵ������ʵ����仯,����ʽ��3A(g)��B(g) ![]() 2C(g)����2����ѧƽ�ⳣ��
2C(g)����2����ѧƽ�ⳣ��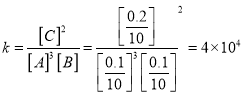 ����3������ͼ��t1ʱ�̲�һ����ƽ��״̬��t1��t2��һ����ƽ���ƶ��������ʼӿ죻�����˴������ӿ췴Ӧ���ʣ���A��ȷ�������˷�Ӧ�¶�����Ӧ���ʼ�������B�����������г�����C��C�����ʵ���Ӧ��ͻ�䣬��ͼ����C�������ʵ����ǽ��䣬��C������С������������ӿ췴Ӧ���ʣ���D��ȷ��
����3������ͼ��t1ʱ�̲�һ����ƽ��״̬��t1��t2��һ����ƽ���ƶ��������ʼӿ죻�����˴������ӿ췴Ӧ���ʣ���A��ȷ�������˷�Ӧ�¶�����Ӧ���ʼ�������B�����������г�����C��C�����ʵ���Ӧ��ͻ�䣬��ͼ����C�������ʵ����ǽ��䣬��C������С������������ӿ췴Ӧ���ʣ���D��ȷ��

 �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�����Ŀ���ش���������
��1����������ʮ�����ʣ���O2����Fe����CaO����CO2����H2SO4����Ba��OH��2���ߺ��ɫ�������������壻����������Һ����ϡ�����Cu2��OH��2CO3 �� ��i�������ʵ���״���෨��д����Ŀհ״���
����� | �������� | ������ | ��Һ | ���� |
���ڸ�������� | �� | ��� |
��ii���������������ڷǵ���ʵ����������������������ᷴӦ�ĵ������������ţ���
��2����i���� KClO3+6HCl��Ũ��=KCl+3Cl2��+3H2O �ķ�Ӧ�У��������� �� ��ԭ������ �� ���������뻹ԭ������������� �� ��ii���ڷ�ӦMnO2+4HCl=MnCl2+Cl2��+2H2O�У�ÿ���ɱ�״����4.48LCl2 �� ת�Ƶĵ��ӵ����ʵ���Ϊmol��
