��Ŀ����
������(AlN)��һ���������ǽ������ϡ�Ϊ�˷���ijAlN��Ʒ����Ʒ�е����ʲ���NaOH��Һ��Ӧ���� AlN�ĺ�����ijʵ��С���������������ʵ�鷽������֪��AlN+NaOH+H2O��NaAlO2+NH3��
������1��ȡһ��������Ʒ��������װ�òⶨ��Ʒ��AlN�Ĵ���(�г�װ������ȥ)��
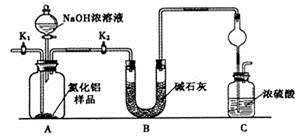
��1����ͼװ���У�U�ι�B����װ����Ϊ________��C�����θ���ܵ�������_______________________��
��2���ر�K1��K2���ٴ�Һ©������������NaOHŨ��Һ�������ٲ������塣��K1��ͨ�뵪��һ��ʱ�䣬�ⶨCװ�÷�Ӧǰ��������仯��ͨ�뵪����Ŀ����_______________________________________��
��3����������װ�û�����____________ȱ�ݣ����²ⶨ���ƫ�ߡ�
������2�������²���ⶨ��Ʒ��AlN�Ĵ��ȣ�

��4����������ɳ��������ӷ���ʽΪ___________________��
��5������۵IJ������õ�����Ҫ����������_________��AlN�Ĵ�����__________����m1��m2��ʾ����
��֪���������Ҫ�ɷ���FeS2����Ԫ�س�+2�ۣ���Ԫ�سʡ�1�ۣ��������Ƿ�����������FeS2���������ַ������������IJ�������ͼ���£�
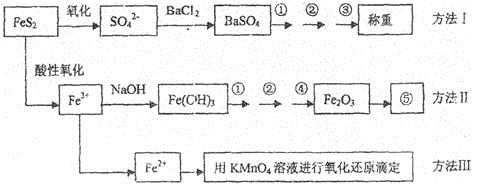
��ͬ���������⣺
(1)����ͼ�в����١��ڡ��۷ֱ�ָ���ǣ���____________����___________����________��
�����ܡ����õ�����Ҫ�����ǣ���_________����__________(ÿ����1��2������)��
(2)�ж���Һ��SO42-���Ӽ�������ȫ�ķ�����______________________________________��
(3)ijͬѧ�÷�����ⶨ������FeԪ�صĺ�������Ҫȷ����KMnO4����Һ���������ص��������Ƶ���ҺŨ��ƫ�����
A���������� | B������ʱ���� |
C������ʱ���ʺ�����λ�÷��ˣ���Ҫ���룩 | D������ƿ�ô�װҺ�� |
(4)ijͬѧ���÷����������ʯ�е�Fe���������ֲⶨ�������ƫ�ߣ���������Ŀ���ԭ����______________________________________��
(5)��ȡ��ʯ����1.60g����������������Ƶ�BaSO4������Ϊ4.66g�������ʯ�е���Ԫ��ȫ��������FeS2����ÿ�ʯ��FeS2������������_________________________��
����ʵ���У�Ϊʵ��ʵ��Ŀ�ı�����ӣ�������ȷ����
ʵ�� | �����Լ� | ʵ��Ŀ�� | |
�� | ��ʯ��ˮ��Ӧ | CuSO4��Һ | ��KMnO4������Һ������Ȳ�Ļ�ԭ�� |
�� | CH3CH2Br��NaOH��Һ���� | HNO3��Һ | ��AgNO3��Һ����CH3CH2Br�е�Br |
�� | ������ϡH2SO4ˮԡ���� | HNO3��Һ | ��������Һ����ˮ�����Ļ�ԭ�� |
�� | C2H5OH��ŨH2SO4������170�� | NaOH��Һ | ��KMnO4��Һ֤���÷�ӦΪ��ȥ��Ӧ |
�� | ����Һ�巴Ӧ | CCl4 | ��AgNO3��Һ֤���÷�ӦΪȡ����Ӧ |
A. �٢ڢۢܢ� B. ֻ�Тڢܢ� C. ֻ�Тڢۢ� D. ֻ�Т٢ڢܢ�