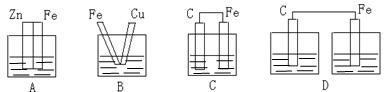
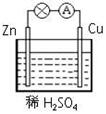
��Ŀ����
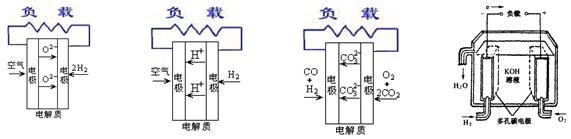
��12�֣���ͼ��һ����ѧ���̵�ʾ��ͼ����֪�׳ص��ܷ�ӦʽΪ��
2CH3OH+3O2+4KOH
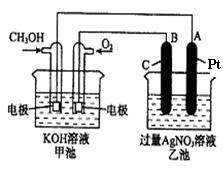
��1����ش�ͼ�мס������ص��й����⣺�׳��� װ�ã�B(ʯī)�缫�������� ��
��2��д���缫��Ӧʽ��ͨ��O2�ĵ缫�ķ�Ӧʽ ��A(Pt)�缫�ķ�ӦʽΪ ��
��3���ҳ��з�Ӧ�Ļ�ѧ����ʽΪ ��
��4�����ҳ���Һ���Ϊ500mL���Һ��Ե���������Һ����ı仯�����ҳ���A������������5.40gʱ��
�ټ׳�������������O2���Ϊ ����״���£���
���ҳ���������Һ��pH= ��
2CH3OH+3O2+4KOH


��1����ش�ͼ�мס������ص��й����⣺�׳��� װ�ã�B(ʯī)�缫�������� ��
��2��д���缫��Ӧʽ��ͨ��O2�ĵ缫�ķ�Ӧʽ ��A(Pt)�缫�ķ�ӦʽΪ ��
��3���ҳ��з�Ӧ�Ļ�ѧ����ʽΪ ��
��4�����ҳ���Һ���Ϊ500mL���Һ��Ե���������Һ����ı仯�����ҳ���A������������5.40gʱ��
�ټ׳�������������O2���Ϊ ����״���£���
���ҳ���������Һ��pH= ��
��1��ԭ��ػ�ѧ��ת��Ϊ���ܵģ�1�֣���������1�֣�
��2��O2+2H2O+4e��="=" 4OH����2�֣�Ag++e��="=" Ag��2�֣�
��3��4AgNO3+2H2O

��4���� 280mL��2�֣����� 1��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
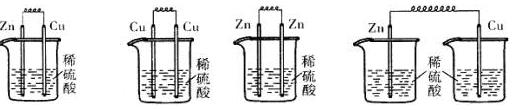
 ��3����ͼ��һ���绯ѧװ��ʾ��ͼ�����¡�������ȼ�ϵ������װ�õĵ�Դ��
��3����ͼ��һ���绯ѧװ��ʾ��ͼ�����¡�������ȼ�ϵ������װ�õĵ�Դ��
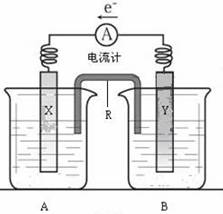
 ���·�е��ӴӸ�����������
���·�е��ӴӸ�����������