��Ŀ����
ʵ���ҽ�I2����KI��Һ�У�����Ũ�Ƚϴ�ĵ�ˮ����Ҫ����Ϊ�����˷�Ӧ��I2(aq)��I��(aq) I(aq)������ƽ����ϵ�У�I�����ʵ���Ũ��c(I)���¶�T�Ĺ�ϵ��ͼ��ʾ(�����ϵ��κ�һ�㶼��ʾƽ��״̬)������ȷ����( )
I(aq)������ƽ����ϵ�У�I�����ʵ���Ũ��c(I)���¶�T�Ĺ�ϵ��ͼ��ʾ(�����ϵ��κ�һ�㶼��ʾƽ��״̬)������ȷ����( )

 I(aq)������ƽ����ϵ�У�I�����ʵ���Ũ��c(I)���¶�T�Ĺ�ϵ��ͼ��ʾ(�����ϵ��κ�һ�㶼��ʾƽ��״̬)������ȷ����( )
I(aq)������ƽ����ϵ�У�I�����ʵ���Ũ��c(I)���¶�T�Ĺ�ϵ��ͼ��ʾ(�����ϵ��κ�һ�㶼��ʾƽ��״̬)������ȷ����( ) 
| A���÷�Ӧ������Ӧ�����ȷ�Ӧ | B���ڷ�Ӧ���е�D��ʱ��v����v�� |
| C��A����C��Ļ�ѧ��Ӧ����vA��vC | D��A����B����ȣ�B���c(I2)�� |
A
�����������ͼ��֪���¶�Խ�ߣ�I��ƽ��Ũ��Խ�ͣ�������Ӧ�Ƿ��ȷ�Ӧ��A��
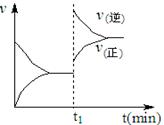
D��ʱIŨ�ȵ���ƽ��Ũ�ȣ���v����v�棬B��
�¶�Խ�ߣ���Ӧ����Խ�죬T1<T2����vA��vC��C��
��A��Bƽ���������ƶ�����B���c(I2)��D��
������������ͼ�黯ѧ��Ӧ���ʺ�ƽ���������⣬����ѧ���Ķ�ͼ������ע��ͼ���е���㣬�յ�����ߵı仯���ƣ��Ѷ�һ��

��ϰ��ϵ�д�
�����Ŀ
 CO(g)+3H2(g) ��H=+206.0kJ?mol��1
CO(g)+3H2(g) ��H=+206.0kJ?mol��1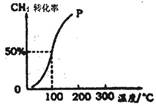

 4NO(g)��6H2O(g)��H����1025 kJ/mol������Ӧ����ʼ���ʵ�����ͬ�����й��ڸ÷�Ӧ��ʾ��ͼ����ȷ����
4NO(g)��6H2O(g)��H����1025 kJ/mol������Ӧ����ʼ���ʵ�����ͬ�����й��ڸ÷�Ӧ��ʾ��ͼ����ȷ����
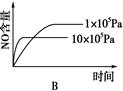
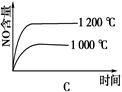
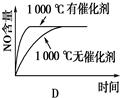
 NH4++NH2����NH4+��ƽ��Ũ��Ϊ1��10��15mol��L��1������˵���������(�� ��)
NH4++NH2����NH4+��ƽ��Ũ��Ϊ1��10��15mol��L��1������˵���������(�� ��) 2C��g��������2 s���룩����C��Ũ��Ϊ0.6 mol·L��1���������м���˵����������ȷ���ǣ����� ��
2C��g��������2 s���룩����C��Ũ��Ϊ0.6 mol·L��1���������м���˵����������ȷ���ǣ����� ��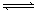 XC���������� 2 s���룩��Ӧ��ƽ��,��� C ��Ũ��Ϊ 0.6 mol��L��1 ��B�����ʵ���Ϊ1.4 mol,�������м���˵����
XC���������� 2 s���룩��Ӧ��ƽ��,��� C ��Ũ��Ϊ 0.6 mol��L��1 ��B�����ʵ���Ϊ1.4 mol,�������м���˵���� 2C(g)������ӦΪ���ȷ�Ӧ������ȷͼ���ǣ� ��
2C(g)������ӦΪ���ȷ�Ӧ������ȷͼ���ǣ� ��
 2SO3 (g) ����H��0
2SO3 (g) ����H��0 2SO3����֪V��SO2��==0.05mol��l-1��min-1����2min��SO3��Ũ��Ϊ�� ��
2SO3����֪V��SO2��==0.05mol��l-1��min-1����2min��SO3��Ũ��Ϊ�� ��