题目内容
【题目】下列图示与对应的叙述不相符合的是
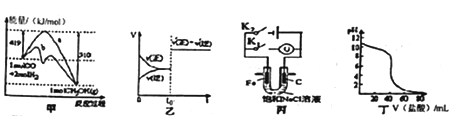
A. 图甲表示工业上用CO生产甲醇的反应CO(g)+2H2(g) ![]() CH3OH(g)。该反应的 △H=-91 kJ·mol-1
CH3OH(g)。该反应的 △H=-91 kJ·mol-1
B. 图乙表示己达平衡的某反应,在t0时改变某一条件后反应速率随时间变化,则改 变的条件可能处加入催化剂
C. 图丙中若K1闭合,石墨棒周围液pH逐渐升高;若K2闭合,电路中通过0.002NA 个电子时,两极理论上共产生0.002mol气体
D. 图丁表示盐酸滴加0.1 mol·L-1NaOH溶液得到的滴定曲线,该实验的指示剂最好选取酚酞
【答案】D
【解析】A、根据图甲,反应物的总能量大于生成物的总能量,此反应是放热反应,因此CO(g)+2H2(g)=CH3OH(g) △H=(419-510)kJ·mol-1=-91kJ·mol-1,故A说法正确;B、根据图乙,t0时刻改变某一因素化学反应速率增大,但平衡不一定移动,t0时刻可能加入催化剂,因此催化剂使反应速率加快,但对化学平衡无影响,故B说法正确;C、闭合K1,构成原电池,发生吸氧腐蚀,铁作负极,石墨作正极,即正极反应式为O2+2H2O+4e-=4OH-,c(OH-)增大,pH升高,闭合K2,构成电解池,石墨为阳极,铁为阴极,阳极反应式为2Cl--2e-=Cl2↑,阴极反应式为2H++2e-=H2↑,通过0.02mol电子,两极产生气体的物质的量为(0.01+0.01)mol=0.02mol,故C说法正确;D、NaOH是强碱,0.1mol·L-1NaOH溶液的pH=13,因此曲线的起点错误,盐酸是强酸,因此强酸滴定强碱,指示剂可以是甲基橙,也可以是酚酞,故D说法错误。

 阅读快车系列答案
阅读快车系列答案【题目】苯甲酸甲酯是重要的化工原料,某化学兴趣小组仿照实验室制乙酸乙酯的原理以苯甲酸(C6H5COOH)和甲醇为原料制备苯甲酸甲酯。有关数据如下:
相对分子质量 | 熔点/℃ | 沸点/℃ | 密度(g/cm3) | 水溶性 | |
苯甲酸 | 122 | 122.4 | 249 | 1.2659 | 微溶 |
甲醇 | 32 | -97 | 64.6 | 0.792 | 互溶 |
苯甲酸甲酯 | 136 | -12.3 | 196.6 | 1.0888 | 不溶 |
I.合成苯甲酸甲酯粗产品
在圆底烧瓶中加入12.2g苯甲酸和20mL甲醇,再小心加入3mL浓硫酸,混匀后,投入几粒碎瓷片,在圆底烧瓶上连接冷凝回流装置后,小心加热2小时,得苯甲酸甲酯粗产品。
回答下列问题:
(1)该反应的化学方程式为__________,该反应的原子利用率是_______。
己知:原子利用率=(预期产物的总质量/全部反应物的总质量)×100%
(2)实验中,应选择(如下图)_____(填序号)作为冷凝回流装置,该仪器的名称为______。

(3)使用过量甲醇的原因是__________。
Ⅱ.粗产品的精制
苯甲酸甲酯粗产品中往往含有少量甲醇、苯甲酸和水等,现拟用下列流程图进行精制。

(4)饱和碳酸钠溶液的作用是________,操作a的名称为________。
(5)由于有机层和水层的密度比较接近,兴趣小组的同学无法直接判断有机层在上层还是下层,请你设计简单易行的方案,简述实验方法,可能的现象及结论__________。
(6)该实验中制得苯甲酸甲酯8.30g,则苯甲酸甲酯的产率为________。
【题目】某同学看到“利用零价铁还原NO3-脱除地下水中硝酸盐”的相关资料后,利用如下装置探究铁粉与KNO3溶液的反应。实验步骤及现象如下:
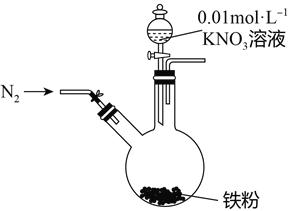
实验步骤 | 实验现象 |
1、打开弹簧夹,缓慢通入N2 | |
2、加入pH为2.5的0.01mol/L酸性KNO3溶液100mL | 铁粉部分溶解,溶液呈浅绿色; 铁粉不再溶解后,剩余铁粉表面出现少量白色物质附着。 |
3、反应停止后,拔掉橡胶塞,将圆底烧瓶取下 | 烧瓶内气体的颜色没有发生变化。 |
4、将剩余固体过滤 | 表面的白色物质变为红褐色。 |
(1)通入N2并保持后续反应均在N2氛围中进行的实验目的是______________________________。
(2)白色物质是__________,用化学方程式解释其变为红褐色的原因:____________________。
(3)为了探宄滤液的成分,该同学进一步设计了下述实验:
实验步骤 | 实验现象 |
1、取部分滤液于试管中,向其中加入KSCN溶液 | 溶液液无变化 |
2、将上述溶液分为两份,一份中滴入氯气;另一份中滴加稀硫酸 | 两份溶液均变为红色 |
3、另取部分滤液于试管中,向其中加入浓NaOH溶液并加热,在试管口放置湿润的红色石蕊试纸。 | 有气体生成,该气体使红色石蕊试纸变蓝。 |
(i)根据以上实验现象,可以判断滤液中存在____________________离子。
(ii)步骤2中滴加稀硫酸后溶液会由浅绿色变成红色,请用离子方程式解释其原因____________________。