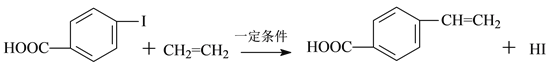��Ŀ����
����Ŀ��I.���Ӻ˴Ź�����(PMR)���о��л���ṹ�������ֶ�֮һ�������о��Ļ����������,ÿһ�ṹ�еĵ�����ԭ����PMR���ж�������Ӧ�ķ�(�ź�),���з��ǿ����ṹ�е�Hԭ���������ȡ����磬��ȩ�ĽṹʽΪCH3CHO,��PMR�����������ź�,��ǿ��֮��Ϊ3:1����֪�л���A�ķ���ʽΪC4H8��
��1�����л���A��PMR��4���źţ���ǿ��֮��Ϊ3:2:2:1,���л��������Ϊ______��
��2�������л���PMRֻ��1���ź�,���ýṹ��ʽ��ʾΪ______��
II����˾��ƽ�������Ƹ�Ѫѹ��һ���ٴ�ҩ��ϳ�����Ч�ɷֵ�ҽҩ�м���Ϊ����ᣬ��ṹ��ʽ��ͼ��![]() ��
��
��3�������ķ���ʽΪ_______�����еĺ�������������Ϊ_______��
��4��һ�������£�1mol�����������______molH2�����ӳɷ�Ӧ��
��5��������ͬ���칹��,ͬʱ�������������Ľṹ��ʽΪ________��
�ٱ�����ֻ������ȡ����
����������������ͭ����Һ��Ӧ����ש��ɫ����
�����Ӻ˴Ź���г��4���ź�,��ǿ��֮��Ϊ3:2:2:1
���𰸡� 1-��ϩ 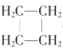 C9H8O2 �Ȼ� 4
C9H8O2 �Ȼ� 4 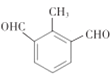 ��
��
����������������ʽΪC4H8���������һ�������Ͷ�����Ҫ��һ��̼̼˫�������γ�һ������
��1�����л���A��PMR��4���źţ���ǿ��֮��Ϊ3:2:2:1��˵������һ����������CH2��һ��CH������Ϊ��CH2=CHCH2CH3������Ϊ��1-��ϩ��
��2�������л���PMRֻ��1���ź�����˵��һ����4����ȫһ����CH2��������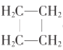 ��
��
��3�������ķ���ʽΪC9H8O2�����еĺ���������Ϊ�Ȼ���
��4��������б������Լӳ�3��H2��˫�����Լӳ�1��H2������1mol�����������4molH2�����ӳɷ�Ӧ��
��5�������ķ���ʽΪC9H8O2����6�������Ͷȡ���ͬ���칹���Ҫ���ǣ��ٱ�����ֻ������ȡ�����������б�����4�������Ͷȣ�����������������ȡ����������������������ͭ����Һ��Ӧ����ש��ɫ������˵������ȩ����-CHO�����������HCOO-���Ļ����ṹ�������Ͷȶ���1���������Ӻ˴Ź���г��4���ź�����ǿ��֮��Ϊ3:2:2:1��˵����һ������-CH3�������ϣ������һ�������Ͷȣ�ֻ����̼��˫�������ݺ˴Ź���Ľ����Ϊ�˱�֤һ���ĶԳ��ԣ�ֻ���DZ�������������ȩ����һ��������ṹΪ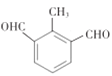 ��
�� ��
��

 С����ȫ�ܼ��ϵ�д�
С����ȫ�ܼ��ϵ�д�����Ŀ��ʵ��������4��ҩƷ�����Ѿ����������Ʒ��
ҩƷ�� | �׳� | �ҳ� | ���� | ���� |
ҩƷ | ���ᣬ���� | �������ƣ��������� | ���ף��� | ͭ��п |
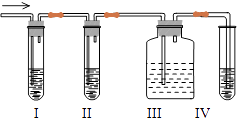
ʵ�����¹���һЩ�⣬Ӧ�ý���Щ�����(����)
A.�׳�B.�ҳ�C.����D.����
����Ŀ�������£���ijһԪ��HA��NaOH��Һ�������ϣ�ʵ����Ϣ���£�
ʵ���� | c(HA)mol��L��1 | c(NaOH)/mol��L��1 | ��Ӧ����ҺpH |
�� | 0.1 | 0.1 | pH=9 |
�� |
| 0.2 | pH=7 |
�����жϲ���ȷ���ǣ� ��
A. 0.1![]() ��HA��Һ����ˮ�������
��HA��Һ����ˮ�������![]()
B. ![]() һ������0.2
һ������0.2![]()
C. ��Ӧ�����Һ�У�HAռ��A��������0.02��
D. �ҷ�Ӧ����Һ�У�c(Na��)��)