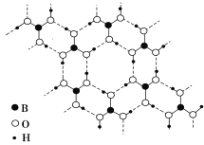
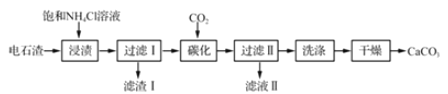
��Ŀ����
����Ŀ���Ե�ʯ��[��Ҫ�ɷ�Ca(0H)2����Fe203��MgO��Si02������]Ϊԭ���Ʊ�����̼��Ƶġ��ֹ����������£�
��1����ʯ������ˮ�γɵ�ʯ����ʱ�ᷢ����Ӧ�����ɵ������г�ˮ���______��
��2�������ա�ʱ��NH4Cl��Ca(0H)2��Ӧ�Ļ�ѧ����ʽΪ______��
��3�������ա�ʱ��һ��ʱ����Ca2+��ȡ�����¶ȱ仯��ͼ��ʾ��Ca2+��ȡ�����¶����߶�������ԭ�������______��
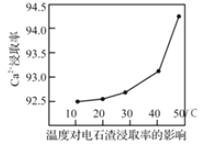
A.�¶����ߣ������ȡ��Ӧ���ʣ��Ӷ����Ca2+��ȡ��
B.�¶����ߣ���Һ���ȼ�С���Ӷ����Ca2+��ȡ��
C.�¶����ߣ�NH4Cl��Һˮ��̶ȼ�С���Ӷ����Ca2+��ȡ��
��4����̼����ʱ��-����õ��¹��գ���Ӧ�����ӷ���ʽΪ___��
��5����Һ���У���ѭ�����õ����ʵĻ�ѧʽΪ___��
��6����ϴ�ӡ�ʱ�������Ƿ�ϴ���ķ�����:__________��
���𰸡�CaSiO3 2NH4Cl��Ca(OH)2===CaCl2��2NH3��H2O AB Ca2���� 2NH3��H2O��CO2===CaCO3����2NH4+ ��H2O��Ca2����2NH3��H2O ��CO2===CaCO3����2NH4+ NH4Cl ȡ�������һ��ˮϴ��Һ���Թ��У���HNO3�ữ������AgNO3��Һ�����ް�ɫ������������ϴ��(�����а�ɫ����������δϴ��)
��������
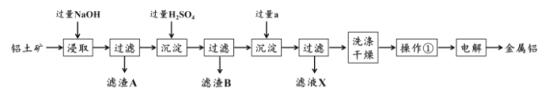
��ʯ��[��Ҫ�ɷ�Ca(OH)2����Fe2O3��MgO��SiO2������]�����뱥���Ȼ�什��գ����ܽ�Ca(OH)2����Fe2O3��MgO������1ΪSiO2��ͨ�������̼��������̼��Ƴ�����������ϴ�Ӹ���ɵõ�̼��Ƴ̶ȣ���Һ��Ϊ��Σ��Դ˽����⡣
(1)��ʯ��[��Ҫ�ɷ�Ca(OH)2����Fe2O3��MgO��SiO2������]����ˮ������Ca(OH)2������ˮ����SiO2Ϊ������������ڼӦ�����κ�ˮ����SiO2�ܺ�Ca(OH)2��Ӧ����CaSiO3��ˮ��
(2)��������ʱ��NH4Cl��Ca(OH)2��Ӧ�����Ȼ��ƺͰ�������ѧ����ʽΪ2NH4Cl+Ca(OH)2�TCaCl2+2NH3H2O��
(3)A.�¶����ߣ�������Ӧ���ʣ��������¶ȣ��ɵ��°����ӷ���ʹƽ�������ƶ�����߸����ӵĽ�ȡ�ʣ���A��ȷ��
B. �¶������ܱ���Һ���ȼ�С���Ӷ����Ca2+��ȡ�ʣ���B��ȷ��
C.�����¶ȣ��ɴٽ�笠�����ˮ�⣬��Һ������ǿ�����Ca2+��ȡ�ʣ���C����
�ʴ�ΪAB��
(4)��̼����ʱ��һ����õ��¹��գ����ٰ����Ļӷ�����ַ�Ӧ������ʽΪCa2++2NH3H2O+CO2�TCaCO3��+2NH4++H2O��Ca2++2NH3+H2O+CO2�TCaCO3��+2NH4+��
(5)��Һ��Ϊ��Σ���Ҫ�ɷ�ΪNH4Cl����ѭ��ʹ�ã�
(6)����������Һ�е������ӣ���ͨ�����������ӵķ������飬������ȡ�������һ��ˮϴ��Һ���Թ��У���HNO3�ữ������AgNO3��Һ�����ް�ɫ������������ϴ��(�����а�ɫ����������δϴ��)��