��Ŀ����
X��Y��Z��������Ԫ�صĵ����ڳ����¶��dz�������ɫ���壬���ʵ������£�����֮���������������Ӧ���ɷֱ���˫�ˡ����˺��ĺ˵ļס��ҡ������ַ��ӣ����ҡ��������к���XԪ�ص�ԭ�Ӹ�����Ϊ2��3����ش��������⣺
(1)Ԫ��X��������________�������ӵĵ���ʽΪ________��
(2)������Y�����ڳ����»�Ͼ�������������Ļ�ѧʽΪ________������һ��������ת��Ϊ���ҵķ�Ӧ����ʽΪ___________________________��
(3)�����ﶡ��X��Y��Z����Ԫ�أ�����һ�ֳ�����ǿ�ᣬ������������ʵ���֮��1��1��Ϻ�����������ľ���ṹ�к��еĻ�ѧ��Ϊ________(ѡ�����)��
a��ֻ�����ۼ� b��ֻ�����Ӽ� c���Ⱥ����Ӽ����ֺ����ۼ�
(1)Ԫ��X��������________�������ӵĵ���ʽΪ________��
(2)������Y�����ڳ����»�Ͼ�������������Ļ�ѧʽΪ________������һ��������ת��Ϊ���ҵķ�Ӧ����ʽΪ___________________________��
(3)�����ﶡ��X��Y��Z����Ԫ�أ�����һ�ֳ�����ǿ�ᣬ������������ʵ���֮��1��1��Ϻ�����������ľ���ṹ�к��еĻ�ѧ��Ϊ________(ѡ�����)��
a��ֻ�����ۼ� b��ֻ�����Ӽ� c���Ⱥ����Ӽ����ֺ����ۼ�
(1)�⡡
(2)NO��4NH3��5O2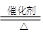 4NO��6H2O
4NO��6H2O
(3)c

(2)NO��4NH3��5O2
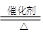 4NO��6H2O
4NO��6H2O(3)c
�ӷ��ӵ��ص�͵���Ϊ��ɫ����ȥ������ȷ����X��Y��Z�ֱ�ΪH��O��N���ס��ҡ����ֱ�ΪNO��H2O��NH3����ΪHNO3����ΪNH4NO3����NH4NO3�����мȺ������Ӽ����ֺ��м��Թ��ۼ���

��ϰ��ϵ�д�
�����Ŀ



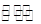 Y2(g)+3Z2(g)����Ӧʱ��(t)��������������ѹǿ(p)�����ݼ��±�
Y2(g)+3Z2(g)����Ӧʱ��(t)��������������ѹǿ(p)�����ݼ��±�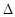 H=+67��7 kJ��mol-1��
H=+67��7 kJ��mol-1��