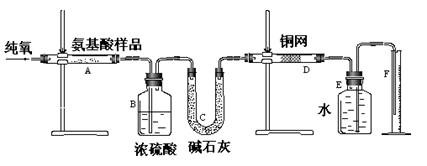
��Ŀ����
(12��)ʵ������һƿ�ܷⲻ�ϵ�Ư�ۣ������������������Լ�����ɸ�Ư�۳ɷֵ�̽�����Թܡ��ιܡ������ܵĵ�����������ˮ������ˮ��1mol/LHCl��Ʒ����Һ�����Ƴ���ʯ��ˮ��
(1) ������裺����һ����Ư��δ���ʣ���________________________��
���������Ư��ȫ�����ʣ���________________________��
����������Ư�۲��ֱ��ʣ���CaCl2 , Ca(ClO)2 ,CaCO3
(2)����ʵ�飺�ڴ��������±�(���ؼ���Ca2+��Cl-)
| | ʵ�鲽�� | Ԥ������ͽ��� |
| �� | ��A�Թ�ȡ��������ʯ��ˮ���ã���B�Թ�ȡ������Ʒ������B�Թܼ���__________ | ��������ų��ҳ���ʯ��ˮδ�����ǣ������________������ |
| �� | | |
| �� | | |
(1) CaCl2��Ca(ClO)2 (1��) CaCl2��CaCO3 (1��) ʵ�鲽�� Ԥ������ͽ��� �� ��A�Թ�ȡ��������ʯ��ˮ���ã���B�Թ�ȡ������Ʒ������B�Թܼ���___1mol/L��ϡ����____(1��) ��������ų��ҳ���ʯ��ˮδ�����ǣ�
�����___һ_____������(1��)�� ʵ�����ݼ����裺(1��)
ȡ������Ʒ���Թ��У�����������ϡ���ᣬ����������������ͨ��Ʒ����Һ�ͳ���ʯ��ˮ����__Ʒ�첻��ɫ��
��ʯ��ˮ�����_________(1��)
���ۣ�����__��___���� (1��)�� ʵ�����ݼ����裺(1��)
ȡ������Ʒ���Թ��У�����������ϡ���ᣬ����������������ͨ��Ʒ����Һ�ͳ���ʯ��ˮ����
Ʒ����Һ��ɫ��
����ʯ��ˮ�����____(1��)��
���ۣ�����������
����

(12��)ʵ������һƿ�ܷⲻ�ϵ�Ư�ۣ� �����������������Լ�����ɸ�Ư�۳ɷֵ�̽�����Թܡ��ιܡ������ܵĵ�����������ˮ������ˮ��1mol/LHCl��Ʒ����Һ�����Ƴ���ʯ��ˮ��
(1) ������裺����һ����Ư��δ���ʣ� ��________________________��
���������Ư��ȫ�����ʣ���________________________��
����������Ư�۲��ֱ��ʣ���CaCl2, Ca(ClO)2 ,CaCO3
(2)����ʵ�飺�ڴ��������±�(���ؼ���Ca2+��Cl-)
|
| ʵ�鲽�� | Ԥ������ͽ��� |
| �� | ��A�Թ�ȡ��������ʯ��ˮ���ã���B�Թ�ȡ������Ʒ������B�Թܼ���________________
| ��������ų��ҳ���ʯ��ˮδ�����ǣ������________������ |
| �� |
|
|
| �� |
|
|
(12��)ʵ������һƿ�ܷⲻ�ϵ�Ư�ۣ� �����������������Լ�����ɸ�Ư�۳ɷֵ�̽�����Թܡ��ιܡ������ܵĵ�����������ˮ������ˮ��1mol/LHCl��Ʒ����Һ�����Ƴ���ʯ��ˮ��
(1) ������裺����һ����Ư��δ���ʣ� ��________________________��
���������Ư��ȫ�����ʣ���________________________��
����������Ư�۲��ֱ��ʣ���CaCl2 , Ca(ClO)2 ,CaCO3
(2)����ʵ�飺�ڴ��������±�(���ؼ���Ca2+��Cl-)
|
|
ʵ�鲽�� |
Ԥ������ͽ��� |
|
�� |
��A�Թ�ȡ��������ʯ��ˮ���ã���B�Թ�ȡ������Ʒ������B�Թܼ���________________
|
��������ų��ҳ���ʯ��ˮδ�����ǣ������________������ |
|
�� |
|
|
|
�� |
|
|