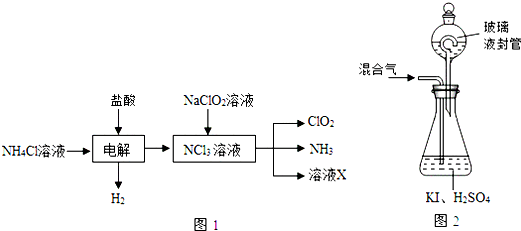
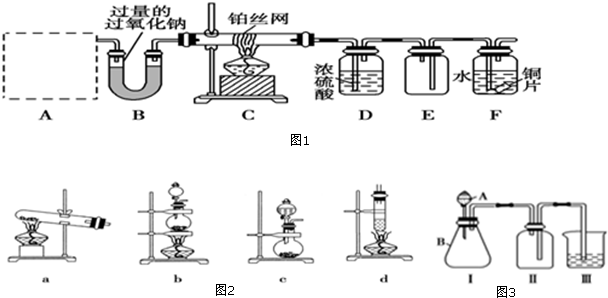
��Ŀ����
3����ش���1��CO2�ĵ���ʽ
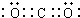 ��
����2����ȼ�ղ�����CO2����û�ѧ����ʽ��ʾ������4Na+CO2$\frac{\underline{\;����\;}}{\;}$C+2Na2O��
��3���ؾ�ʯ��BaSO4���������ᣬ�ñ���Na2CO3����ת��Ϊ���������BaCO3��д����Ӧ�����ӷ���ʽBaSO4 ��s��+CO32-��aq��=BaCO3��s��+SO42-��aq����
��4���������������ԭ��Ӧ�����ӷ���ʽ��
1Cr2O72-+3H2O2+8H+�T2Cr3++3O2��+7H2O��
���� ��1��CO2�ǹ��ۻ������ṹʽΪO=C=O��̼ԭ�Ӻ���ԭ��֮����2�Ե��ӣ�
��2�������£����������̼��Ӧ������ȼ�������ƣ�
��3��BaSO4 ת��ΪBaCO3�����ӷ���ʽΪBaSO4 ��s��+CO32-��aq��=BaCO3��s��+SO42-��aq����
��4���÷�Ӧ�У��䷽��ʽΪH2CrO4���������������������ǻ�ԭ�������ݵ�ʧ���Ӻ͵���غ���ƽ��
��� �⣺��1��CO2�ǹ��ۻ������ṹʽΪO=C=O��̼ԭ�Ӻ���ԭ��֮����2�Ե��ӣ������ʽΪ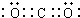 ��
��
�ʴ�Ϊ��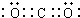 ��
��
��2�������£����������̼��Ӧ���������ƣ���ѧ��Ӧ����ʽΪ��4Na+CO2$\frac{\underline{\;����\;}}{\;}$C+2Na2O���������������ã��ʴ�Ϊ��4Na+CO2$\frac{\underline{\;����\;}}{\;}$C+2Na2O��
��3��BaSO4 ת��ΪBaCO3�����ӷ���ʽΪBaSO4 ��s��+CO32-��aq��=BaCO3��s��+SO42-��aq�����ʴ�Ϊ��BaSO4 ��s��+CO32-��aq��=BaCO3��s��+SO42-��aq������4���÷�Ӧ�У��䷽��ʽΪH2CrO4���������������������ǻ�ԭ�������ݵ�ʧ���Ӻ͵���غ���ƽ��Cr2O72-+3H2O2+8H+�T2Cr3++3O2��+7H2O���ʴ�Ϊ��1��3��8H+��2��3��7H2O��
���� ������Ҫ������dz���Ԫ���Լ��仯�����֪ʶ���ۺ��Խ�ǿ���漰����ʽ����д�����ʵ��жϡ�������ԭ��Ӧ���ǵ����غ㡢��ѧ��Ӧ����ʽ��д��������ԭ�����ӷ���ʽ����ƽ�ȣ��ѶȽϴ�

����������ˮ�����ڻ����
��AlCl3��Һ����������Һ��������Һ
��SiO2��CO����������������
�ܺ���ԭ�ӵ��β�һ��������ʽ�Σ�
����˵������ȷ���ǣ�������
| A�� | �٢� | B�� | �٢� | C�� | �ڢ� | D�� | �ۢ� |
| A�� | 1 mol Fe2+��������H2O2��Һ��Ӧ��ת��2NA������ | |
| B�� | �����ʵ�����N2��CO����ԭ������ͬ��������ͬ | |
| C�� | �ڱ���£�22.4 L SO3������ԭ��Ϊ3NA | |
| D�� | 1L 0.1 mol•L-1��������Һ��H+��Ϊ0.1 NA |
| A�� | ${\;}_{8}^{16}$O2��${\;}_{8}^{18}$O2��Ϊͬλ�أ����������в������ѧ�������� | |
| B�� | N2��O2�ķ�Ӧ���ڵ��Ĺ̶� | |
| C�� | Ԫ�����ڱ���7�����зֱ���7�����ڣ�18�����зֱ���18���� | |
| D�� | 1mol���ʯ��1molʯīȼ�շų������࣬���Խ��ʯ��ʯī�ȶ� |
| A�� | 32 g | B�� | 32 g/mol | C�� | 34 g | D�� | 34 g/mol |