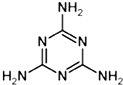
��Ŀ����
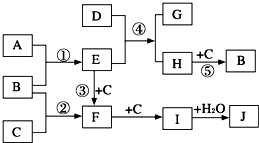 ��2009?����һģ����ͼ�Dz��ֶ�����Ԫ�صĵ��ʼ��仯�����ת����ϵͼ���йط�Ӧ�����������ɵ�H2O����ȥ����
��2009?����һģ����ͼ�Dz��ֶ�����Ԫ�صĵ��ʼ��仯�����ת����ϵͼ���йط�Ӧ�����������ɵ�H2O����ȥ������֪����a��A��B��CΪ���ʣ����ڳ��³�ѹ��Ϊ���壻
��b����Ӧ�٢�Ϊ���������е���Ҫ��Ӧ������Ϊ��������ʱ�����ķ�Ӧ��
��c��������D����Ư���ԣ�����C12��NaOH��aq����Ӧ���Ƶã�
��d��������H������Ԫ����ɣ�����Է�������Ϊ32��
��ش��������⣺?
��1����Ӧ�ٵ�������
���¡���ѹ������
���¡���ѹ������
�����з���ƽ���ƶ�ԭ���ķ�Ӧ��������ѹ������
��ѹ������
?��2������������
I
I
������ĸ��ţ�����ɹ⻯ѧ��������Ҫ���أ�?��3����Ӧ���У�ÿ��0.2molE��ȫ��Ӧ�������ת��Ϊ
1
1
mol��?��4��E��H�Ľṹ���������ƣ���Ԥ��H��ˮ��Һ��pH7�������������=����������ԭ����
N2H4+2H2O?N2H62++2OH-
N2H4+2H2O?N2H62++2OH-
�������ӷ���ʽ��ʾ�����ڹ�̬ʱ��HΪ����
����
���壬��е��E��
��
����ߡ��͡����� ��5����Ӧ�ݵĻ�ѧ����ʽΪ
N2H4+O2
N2+2H2O
| ||
N2H4+O2
N2+2H2O
��
| ||
������A��B��CΪ���ʣ����ڳ��³�ѹ��Ϊ���壬��Ϊ��������ʱ�����ķ�Ӧ������ǵ�������������NO�ķ�Ӧ������F��NO��A�����嵥�ʣ�A��B��Ӧ����E��E�ܺ�C��Ӧ����NO���ҷ�Ӧ��Ϊ���������е���Ҫ��Ӧ����E��NH3��C��O2��B��N2��A��H2��NO��������������I��IΪNO2��NO2��ˮ��Ӧ���������һ����������J��HNO3��
������D����Ư���ԣ�����C12��NaOH��aq����Ӧ���Ƶã�����Ԫ���غ�֪��D��Na2O2��������H������Ԫ����ɣ�����Է�������Ϊ32��H��������Ӧ���ɵ�������H����Է���������32�����Ԫ���غ�֪��HΪN2H4����GΪNaOH��
������D����Ư���ԣ�����C12��NaOH��aq����Ӧ���Ƶã�����Ԫ���غ�֪��D��Na2O2��������H������Ԫ����ɣ�����Է�������Ϊ32��H��������Ӧ���ɵ�������H����Է���������32�����Ԫ���غ�֪��HΪN2H4����GΪNaOH��
����⣺A��B��CΪ���ʣ����ڳ��³�ѹ��Ϊ���壬��Ϊ��������ʱ�����ķ�Ӧ������ǵ�������������NO�ķ�Ӧ������F��NO��A�����嵥�ʣ�A��B��Ӧ����E��E�ܺ�C��Ӧ����NO���ҷ�Ӧ��Ϊ���������е���Ҫ��Ӧ����E��NH3��C��O2��B��N2��A��H2��NO��������������I��IΪNO2��NO2��ˮ��Ӧ���������һ����������J��HNO3��
������D����Ư���ԣ�����C12��NaOH��aq����Ӧ���Ƶã�����Ԫ���غ�֪��D��Na2O2��������H������Ԫ����ɣ�����Է�������Ϊ32��H��������Ӧ���ɵ�������H����Է���������32�����Ԫ���غ�֪��HΪN2H4����GΪNaOH��
��1�������������ڸ��¡���ѹ�������������·�����Ӧ���ɰ������÷�Ӧ��һ����Ӧǰ�����������С�ķ��ȵĿ��淴Ӧ���¶Ⱥ�ѹǿ��ƽ�ⶼ��Ӱ�죬���Է���ƽ���ƶ�ԭ���������Ǹ�ѹ����ѹ��
�ʴ�Ϊ�����¡���ѹ����������ѹ�����£�?
��2����������������⻯ѧ��������Ҫ���أ���ѡ��
��3����Ӧ���У�ÿ��0.2molNH3��ȫ��Ӧ�������ת�Ƶ����ʵ���=0.2mol����2+3��=1mol��
�ʴ�Ϊ��1��
��4��NH3��N2H4�Ľṹ���������ƣ����ݰ���������֪��H��ˮ��ҺPH��7��ԭ����N2H4+2H2O?N2H62++2OH-��������Һ������������Ũ�ȴ���������Ũ�ȶ�ʹ��Һ�ʼ��ԣ�N2H4��ֻ�����ۼ����乹�����Ƿ��ӣ��������ڷ��Ӿ��壬��е�Ȱ����ĸߣ�ԭ����N2H4��Է����������ڰ������ҷ��Ӽ�Ҳ���γ������
�ʴ�Ϊ������N2H4+2H2O?N2H62++2OH-�����ӣ��ߣ�
��5����ȼ�����ɵ�����ˮ����Ӧ����ʽΪ��N2H4+O2
N2+2H2O��
�ʴ�Ϊ��N2H4+O2
N2+2H2O��?
������D����Ư���ԣ�����C12��NaOH��aq����Ӧ���Ƶã�����Ԫ���غ�֪��D��Na2O2��������H������Ԫ����ɣ�����Է�������Ϊ32��H��������Ӧ���ɵ�������H����Է���������32�����Ԫ���غ�֪��HΪN2H4����GΪNaOH��
��1�������������ڸ��¡���ѹ�������������·�����Ӧ���ɰ������÷�Ӧ��һ����Ӧǰ�����������С�ķ��ȵĿ��淴Ӧ���¶Ⱥ�ѹǿ��ƽ�ⶼ��Ӱ�죬���Է���ƽ���ƶ�ԭ���������Ǹ�ѹ����ѹ��
�ʴ�Ϊ�����¡���ѹ����������ѹ�����£�?
��2����������������⻯ѧ��������Ҫ���أ���ѡ��
��3����Ӧ���У�ÿ��0.2molNH3��ȫ��Ӧ�������ת�Ƶ����ʵ���=0.2mol����2+3��=1mol��
�ʴ�Ϊ��1��
��4��NH3��N2H4�Ľṹ���������ƣ����ݰ���������֪��H��ˮ��ҺPH��7��ԭ����N2H4+2H2O?N2H62++2OH-��������Һ������������Ũ�ȴ���������Ũ�ȶ�ʹ��Һ�ʼ��ԣ�N2H4��ֻ�����ۼ����乹�����Ƿ��ӣ��������ڷ��Ӿ��壬��е�Ȱ����ĸߣ�ԭ����N2H4��Է����������ڰ������ҷ��Ӽ�Ҳ���γ������
�ʴ�Ϊ������N2H4+2H2O?N2H62++2OH-�����ӣ��ߣ�
��5����ȼ�����ɵ�����ˮ����Ӧ����ʽΪ��N2H4+O2
| ||
�ʴ�Ϊ��N2H4+O2
| ||
���������⿼�������ʵ����ʺ����ʼ��ת������ȷ�ƶ������ǽⱾ��ؼ����Է�Ӧ��Ϊͻ�ƿڲ����������ϵķ��������ƶϣ�����������Ϣ��������Ѷ��еȣ�

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ