��Ŀ����
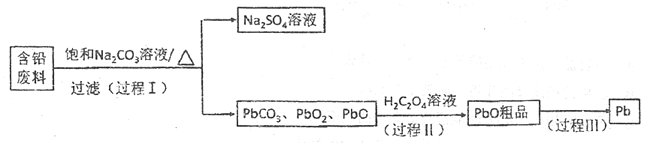
����Ŀ���Ӻ�Ǧ����(PbSO4��PbO2��PbO��)�л���Ǧ��ʵ��Ǧ�����������ش�һ�ֻ���Ǧ�Ĺ����������£�
(1)Ǧ��̼Ԫ��ͬ���壬��̼��4�����Ӳ㣬��ǦԪ�ص�ԭ������Ϊ______��Ǧ���طŵ�ԣ�PbO2��______����
(2)���̢���֪��PbSO4��PbCO3���ܽ�ȣ�20�棩��ͼ1����������ܽ�ȼ�ͼ2��

ͼ1 ͼ2
�ٸ���ͼ1д�����̢�����ӷ���ʽ�� __________________��
�����������е��¶�Ӧ������40�棬���¶Ƚ��ͣ�PbSO4��ת�������½�������ͼ2������ԭ��
i���¶Ƚ��ͣ���Ӧ���ʽ��ͣ�
ii��___________________���������һ�ֺ������ͣ�
(3)���̢�����Ӧ2PbO2+H2C2O4=2PbO+H2O2+2CO2��
��д������ĵ���ʽ________��PbO��Al2O3�������ƣ�PbO������������Һ��Ӧ�����ӷ���ʽ��______________________��
(4)���̢�PbO��Ʒ�ܽ���H2SO4��NaCl�Ļ����Һ�У��õ���Na2PbCl4�ĵ��Һ�����Na2PbCl4��Һ����Pb����ͼ��

�������ĵ缫��Ӧʽ��_____________________��
�ڵ��һ��ʱ���Na2PbCl4Ũ�ȼ����½���Ϊ����ʹ�����̳������У���������ȡ�ķ�����____________��
���𰸡�82 �� PbSO4(s)+CO32-(aq)![]() PbCO3(s)+SO42-(aq) �¶Ƚ��ͣ�̼���Ƶ��ܽ�ȼ�С������Ũ�ȼ�С��ʹ��Ӧ���ʼ�С
PbCO3(s)+SO42-(aq) �¶Ƚ��ͣ�̼���Ƶ��ܽ�ȼ�С������Ũ�ȼ�С��ʹ��Ӧ���ʼ�С  PbO+2OH-=PbO22-+H2O PbCl42-+2e-=Pb+4Cl- ����������PbO��Ʒ
PbO+2OH-=PbO22-+H2O PbCl42-+2e-=Pb+4Cl- ����������PbO��Ʒ
��������
��Ǧ���ϣ�PbSO4��PbO2��PbO�ȣ������뱥��̼������Һ���˵õ�PbCO3��PbO2��PbO����Һ��������Һ��PbCO3��PbO2��PbO���������˵õ�PbO��Ʒ����PbO��Ʒ�ܽ���HCl��NaCl�Ļ����Һ�У��õ���Na2PbCl4�ĵ��Һ�����Na2PbCl4��Һ������Pb��O2��
(1)Pb��Cλ��ͬһ���壬��C���ĸ����ڣ���Pb�ǵ������ڵ�IVA��Ȼ����ԭ�ӽṹ��Ԫ��λ�ù�ϵ�ж�ǦԪ�ص�ԭ��������Ǧ���طŵ�ʱPb��������PbO2��������
(2)��̼��Ǧ�ܽ��С������Ǧ��
��i.��Ӧ���ʼ�С��ԭ�����¶Ƚ��͡�����Ũ�ȼ�С��
ii.�¶ȵ���40��ʱ���¶Ƚ��ͣ��¶Ƚ��ͣ�̼���Ƶ��ܽ�ȼ�С������Ũ�ȼ�С��ʹ��Ӧ���ʼ�С��
(3)������ӽṹ��ʽΪHOOC-COOH���ɸ�����ṹ��ʽ�õ������ʽ��PbO��������������NaOH��Ӧ����Na2PbO2��ˮ��
(4)���Na2PbCl4��Һ������Pb��O2��
�������ĵ缫��Ӧ�Ƿ�����ԭ��Ӧ��Ԫ�ػ��ϼ۽��ͣ�
���������һ��ʱ�����ҺΪHCl��NaC1�Ļ����Һ��������������PbO��Ʒ�ܽ���HCl��NaC1�Ļ����Һ�У��õ���Na2PbC14�ĵ��Һ����֪�����PbO��
��Ǧ���ϣ�PbSO4��PbO2��PbO�ȣ������뱥��̼������Һ���˵õ�PbCO3��PbO2��PbO����Һ��������Һ��PbCO3��PbO2��PbO���������˵õ�PbO��Ʒ����PbO��Ʒ�ܽ���HCl��NaCl�Ļ����Һ�У��õ���Na2PbCl4�ĵ��Һ�����Na2PbCl4��Һ������Pb��O2��
(1)Ǧ��̼Ԫ��ͬ���壬��̼��4�����Ӳ㣬��Pb�ǵ������ڵ�IVA��Ԫ�أ�����ͬһ����ԭ��������������ڰ�����Ԫ��������Ŀ��ϵ��֪Pbԭ������Ϊ82��Ǧ���طŵ�ʱ��ǦԪ�ػ��ϼ�0�۱仯Ϊ+2�ۣ�Pb��������ǦԪ�ػ��ϼ�+4�۱仯Ϊ+2�ۣ�PbO2��������
(2)�ٸ���ͼʾ��֪��̼��Ǧ�ܽ��С������Ǧ�������ܶȻ������֪���ܽ�Ƚ�С��������������ܽ�ϴ��������ת�����ʸ÷�Ӧ�ǿ��淴Ӧ������I�����ӷ���ʽΪ��PbSO4(s)+CO32-(aq)![]() PbCO3(s)+SO42-(aq)��
PbCO3(s)+SO42-(aq)��
�����������е��¶�Ӧ������40�棬���¶Ƚ��ͣ�PbSO4��ת�������½�����Ӧ���ʼ�С����һ��ԭ�����¶Ƚ��ͣ�̼���Ƶ��ܽ�ȼ�С������Ũ�ȼ�С��ʹ��Ӧ���ʼ�С��
(3)������ӽṹ��ʽΪHOOC-COOH�������ʽ�ɱ�ʾΪ�� �� PbO�������������NaOH��Ӧ����Na2PbO2��ˮ����Ӧ�����ӷ���ʽ��PbO+2OH-=PbO22-+H2O��
�� PbO�������������NaOH��Ӧ����Na2PbO2��ˮ����Ӧ�����ӷ���ʽ��PbO+2OH-=PbO22-+H2O��
(4)���Na2PbCl4��Һ������Pb��O2��
�������ĵ缫��Ӧ�Ƿ�����ԭ��Ӧ��Ԫ�ػ��ϼ۽��ͣ������ĵ缫��Ӧʽ�ǣ�PbCl42-+2e-=Pb+4Cl-��
���������һ��ʱ�����ҺΪHCl��NaC1�Ļ����Һ��������������PbO��Ʒ�ܽ���HCl��NaC1�Ļ����Һ�У��õ���Na2PbC14�ĵ��Һ�� Ϊ����ʹ�����̳������У���������������PbO��Ʒ�ɻָ���Ũ����ʵ�����ʵ�ѭ�����á�

 ��У����ϵ�д�
��У����ϵ�д�



