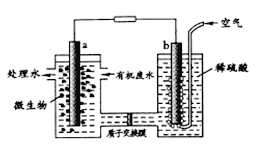
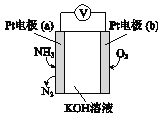
��Ŀ����
����Ŀ��A��B��C��D 4��Ԫ�أ�����A��B��CΪ������Ԫ�أ�AԪ��������������������������ԭ����������ȣ�B��ԭ�Ӱ뾶����������������С�ģ�B������������Ӧˮ����Ļ�ѧʽΪHBO3��CԪ��ԭ�ӵ������������ȴ������2����C����������D�������Ӿ�����ͬ�ĵ����Ų�����Ԫ�ؿ��γɻ�����D2C��
��1��BԪ�ص����� ��B�����ڱ��е�λ�õ� ���ڣ��� �壻
��2��A��B�γɵĻ�����ĵ���ʽ ��
��3��C��Ԫ�ط��� ��C�����������Ļ�ѧʽ ��
��4��D������������Ӧ��ˮ����Ļ�ѧʽ ��
���𰸡���1��������������A�壨2��![]() ��3��S��SO3��4��KOH
��3��S��SO3��4��KOH
�����������������AԪ��������������������������ԭ����������ȣ�ӦΪHԪ�أ�B��ԭ�Ӱ뾶����������������С�ģ�B������������Ӧˮ����Ļ�ѧʽΪHBO3����BΪ�ڶ����ڵ���A�壬ΪNԪ��;CԪ��ԭ�ӵ������������ȴ������2����ӦΪSԪ�أ�C����������D�������Ӿ�����ͬ�ĵ��Ӳ�ṹ����Ԫ�ؿ��γɻ�����D2C����DΪKԪ�أ�C��SԪ�ء���1��BΪNԪ�أ�ԭ������Ϊ7��ԭ�Ӻ�����2�����Ӳ㣬����������λ5����λ�ڵڶ����ڵ���A�壬
��2��A��B�γɵĻ�����ΪNH3�����ӵ����幹��Ϊ�����Σ�����ʽΪ![]() ����3��C��Ԫ�ط���S�� C�����������Ļ�ѧʽSO3��
����3��C��Ԫ�ط���S�� C�����������Ļ�ѧʽSO3��
��4��D������������Ӧ��ˮ����Ļ�ѧʽKOH��

 ��������ϵ�д�
��������ϵ�д�