��Ŀ����
����Ŀ��X��Y��Z��R��W ��Ϊ���ڱ���ǰ�����ڵ�Ԫ�أ���ԭ��������������X2-�� Y+���� ͬ�ĺ�������Ų���Z ���⻯��ķе������һ����ͬ��Ԫ���⻯��ķе�ͣ�R �Ļ� ̬ԭ����ǰ������Ԫ�صĻ�̬ԭ���е���������ࣻW Ϊ����Ԫ�أ�X �� W �γɵ�ij �ֻ������� Z ���⻯���Ũ��Һ����ʱ��Ӧ������ʵ������ȡ Z ����̬���ʡ��ش��� �����⣨��ػش����Ԫ�ط��ű�ʾ����
(1)R �Ļ�̬ԭ�ӵĺ�������Ų�ʽ��__________________��
(2)Z ���⻯��ķе������һ����ͬ��Ԫ���⻯��ķе�͵�ԭ����______________��
(3)X �� Z �е縺�Խϴ����_____ ��Z ��ij�ֺ������γ�����ʵ������ X �ĵ��ʵ� ��ȡ����������ӵĿռ乹����______________���������к��еĻ�ѧ������ ��_________ ��X��Z��X �ļ���_______109.5�㣨���������=����������
(4)X �� Y �γɵĻ����� Y2X �ľ�����ͼ������ X ���ӵ���λ�� Ϊ___________�������һ�� X ������������� Y ����Ϊ���㹹�ɵļ�����Ϊ___________ ���û������� MgO ��ȣ��۵�ϸߵ���____________��
(5)��֪�û�����ľ����߳�Ϊ a pm����û�������ܶ�Ϊ________________g��cm-3��ֻҪ������ʽ�����ؼ������ֵ�������ӵ��곣������ֵΪ NA����
���𰸡�![]() HF���Ӽ�����������HCl���Ӽ䲻������� O ������ ���ۼ� �� 8 ������ MgO
HF���Ӽ�����������HCl���Ӽ䲻������� O ������ ���ۼ� �� 8 ������ MgO ![]()
��������
X��Y��Z��R��W��Ϊ���ڱ���ǰ�����ڵ�Ԫ�أ���ԭ��������������x2-��Y+����ͬ�ĺ�������Ų�����X��Y����һ���ڣ���Xλ�ڵ�VIA�塢Yλ�ڵ�IA�壻Z���⻯��ķе������һ����ͬ��Ԫ���⻯��ķе�ͣ���Z�⻯���в������������һ����ͬһ����Ԫ���⻯���������R�Ļ�̬ԭ����ǰ������Ԫ�صĻ�̬ԭ���е���������࣬��RΪCrԪ�أ�Xλ�ڵڶ����ڣ�ΪOԪ�أ�Y��Zλ�ڵ������ڣ�YΪNa��WΪ����Ԫ�أ�X��W�γɵ�ij�ֻ�������Z���⻯���Ũ��Һ����ʱ��Ӧ������ʵ������ȡZ����̬���ʣ�ʵ������Ũ����Ͷ���������ȡ����������WΪMn��ZΪCl���ݴ˷�����
(1) RΪCrԪ�أ���ԭ�Ӻ�����24�����ӣ����ݹ���ԭ����дR�Ļ�̬ԭ�ӵĺ�������Ų�ʽ![]() ��
��
(2) ZΪClԪ�أ��⻯����۷е�����Է������������ȣ�������������⻯���۷е�ϸߣ�HCl���Ӽ䲻�������HF���Ӽ������������HF�۷е����HCl����Ϊ��HF���Ӽ�����������HCl���Ӽ䲻���������
(3) X��O��Z��Cl��O��Cl�е縺�Խϴ����O��ʵ���ҳ�������طֽ���ȡ������![]() ������ԭ�Ӽ۲���Ӷ���Ϊ
������ԭ�Ӽ۲���Ӷ���Ϊ![]() ���Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ��������жϴ�������ӵĿռ乹��Ϊ�����Σ��������к��еĻ�ѧ�������ǹ��ۼ������ڹµ��ӶԺͳɼ����Ӷ�֮����ų������ڳɼ����Ӷ�֮����ų��������������С��109.5�㣻��Ϊ��O�������Σ����ۼ�������
���Һ���һ���µ��Ӷԣ����ݼ۲���ӶԻ��������жϴ�������ӵĿռ乹��Ϊ�����Σ��������к��еĻ�ѧ�������ǹ��ۼ������ڹµ��ӶԺͳɼ����Ӷ�֮����ų������ڳɼ����Ӷ�֮����ų��������������С��109.5�㣻��Ϊ��O�������Σ����ۼ�������
(4)���ݾ�̯����֪��ͼ�п�����ĸ���Ϊ![]() ��ʵ����ĸ���Ϊ8�����Կ�������������ӣ�ʵ������������ӣ�����Na2O�ľ����ṹͼ��֪����������Χ����������Ӹ���Ϊ8������λ��Ϊ8�������һ�����������������������Ϊ���㹹�ɵļ�����Ϊ�����壻���������ӵİ뾶����þ���ӣ������С��þ���ӣ����������Ƶľ�����С������þ����������þ���۵���������ƣ���Ϊ��8�������壻MgO��
��ʵ����ĸ���Ϊ8�����Կ�������������ӣ�ʵ������������ӣ�����Na2O�ľ����ṹͼ��֪����������Χ����������Ӹ���Ϊ8������λ��Ϊ8�������һ�����������������������Ϊ���㹹�ɵļ�����Ϊ�����壻���������ӵİ뾶����þ���ӣ������С��þ���ӣ����������Ƶľ�����С������þ����������þ���۵���������ƣ���Ϊ��8�������壻MgO��
(5)һ������������Ϊ![]() ���������ܶ�
���������ܶ�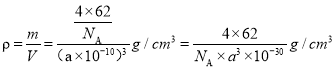 ��
��

 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�����Ŀ����һ���2L���ܱ������м��뷴Ӧ��N2��H2���������·�Ӧ��N2(g)��3H2(g)![]() 2NH3(g)����Ӧ�����еIJ����������±���ʾ������˵����ȷ����
2NH3(g)����Ӧ�����еIJ����������±���ʾ������˵����ȷ����
���ʵ���/ mol ʱ��/min | n(N2) | n(H2) | n(NH3) |
0 | 1.0 | 1.2 | 0 |
2 | 0.9 | ||
4 | 0.75 | ||
6 | 0.3 |
A. 0��2 min�ڣ�NH3�ķ�Ӧ����Ϊ0.1 mol��L��1��min��1
B. 2 minʱ�� H2�����ʵ���0.3 mol
C. 4 minʱ����Ӧ�Ѵﵽƽ��״̬����ʱ�����淴Ӧ�����ʶ�Ϊ0
D. 4��6 min�ڣ�������������ӵ������ʵ�������