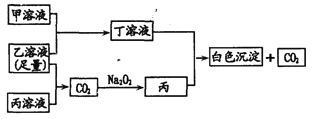
��Ŀ����
����Ŀ����1����þ�����Ļ���ﹲ4.0�ˣ���200��������ǡ����ȫ��Ӧ���ڱ�״���¹��ռ���2.24L���塣���跴Ӧ����Һ������䣬����������Ϊ_____g����Ӧ����Һ��Mg2+�����ʵ���Ũ��Ϊ_______��
��2��һ����������������ȼ�գ����û�������� 100 mL��2.00 moL��L-1NaOH ��Һǡ����ȫ���գ������Һ�к��� NaClO �����ʵ���Ϊ 0.05mol���Լ����������⣺ ������Һ�� Cl-�����ʵ���Ϊ_______ �����������Ͳμӷ�Ӧ�����������ʵ���֮��n��C12����n��H2��Ϊ _______ ��
���𰸡� 2.8 0.25mol�� L��1 0.15 mol 2:1
��������������������⿼������ļ������漰Mg��Fe����ķ�Ӧ��Cl2��H2��NaOH�ķ�Ӧ��
��1�����ݷ�Ӧ�Ļ�ѧ����ʽ��Mg+2HCl=MgCl2+H2����Fe+2HCl=FeCl2+H2������ʽ��n��Mg��+n��Fe��=![]() ��24g/moln��Mg��+56g/moln��Fe��=4.0g�����n��Mg��=0.05mol��n��Fe��=0.05mol������������m��Fe��=0.05mol
��24g/moln��Mg��+56g/moln��Fe��=4.0g�����n��Mg��=0.05mol��n��Fe��=0.05mol������������m��Fe��=0.05mol![]() 56g/mol=2.8g������Mg�غ���n��Mg2+��=n��Mg��=0.05mol��c��Mg2+��=
56g/mol=2.8g������Mg�غ���n��Mg2+��=n��Mg��=0.05mol��c��Mg2+��=![]() =0.25mol/L��
=0.25mol/L��
��2��H2��Cl2��ȼ������HCl��HCl��NaOH��Һ�ķ�ӦΪ��HCl+NaOH=NaCl+H2O����NaOH��Һ���պ�������Һ�к�NaClO��˵��H2��Cl2��ȼ�պ�Cl2����������Cl2��NaOH��Һ�����ķ�ӦΪ��Cl2+2NaOH=NaCl+NaClO+H2O������Na��Cl�غ�����Ӧ����Һ��n��NaCl��+n��NaClO��=n��NaOH��=2mol/L![]() 0.1L=0.2mol��n��NaClO��=0.05mol����n��NaCl��=0.2mol-0.05mol=0.15mol��������Һ��Cl-���ʵ���Ϊ0.15mol������Cl�غ�������Cl2���ʵ���Ϊ��0.15mol+0.05mol��
0.1L=0.2mol��n��NaClO��=0.05mol����n��NaCl��=0.2mol-0.05mol=0.15mol��������Һ��Cl-���ʵ���Ϊ0.15mol������Cl�غ�������Cl2���ʵ���Ϊ��0.15mol+0.05mol��![]() 2=0.1mol������0.05molNaClO��ͬʱ����0.05molNaCl������HCl��NaOH��Ӧ���ɵ�NaClΪ0.15mol-0.05mol=0.1mol��HCl���ʵ���Ϊ0.1mol�����ݷ�ӦH2+Cl2
2=0.1mol������0.05molNaClO��ͬʱ����0.05molNaCl������HCl��NaOH��Ӧ���ɵ�NaClΪ0.15mol-0.05mol=0.1mol��HCl���ʵ���Ϊ0.1mol�����ݷ�ӦH2+Cl2![]() 2HCl���μӷ�Ӧ��H2���ʵ���Ϊ0.05mol������Cl2��μӷ�Ӧ��H2���ʵ���֮��n��Cl2����n��H2��=0.1mol��0.05mol=2:1��
2HCl���μӷ�Ӧ��H2���ʵ���Ϊ0.05mol������Cl2��μӷ�Ӧ��H2���ʵ���֮��n��Cl2����n��H2��=0.1mol��0.05mol=2:1��

 �Ķ��쳵ϵ�д�
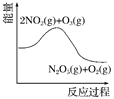
�Ķ��쳵ϵ�д�����Ŀ����������������������Լ�����������ӦΪ2NO2(g)��O3(g) ![]() N2O5(g)��O2(g)������Ӧ�ں����ܱ������н��У������ɸ÷�Ӧ���ͼ���������ж���ȷ����(����)
N2O5(g)��O2(g)������Ӧ�ں����ܱ������н��У������ɸ÷�Ӧ���ͼ���������ж���ȷ����(����)
A | B | C | D |
|
|
|
|
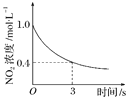
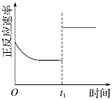
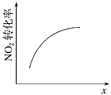
�����¶ȣ�ƽ�ⳣ����С | 0��3 s�ڣ���Ӧ����Ϊv(NO2)�� 0.2 mol��L��1 | t1ʱ�����������ƽ�������ƶ� | �ﵽƽ��ʱ�����ı�x����xΪc(O2) |
A. A B. B C. C D. D
����Ŀ������ͬ�¶��£������Ϊ1 L���ĸ��ܱ������У������¶Ⱥ��ݻ����䣬�����ֲ�ͬ��Ͷ�Ϸ�ʽ���з�Ӧ��ƽ��ʱ�й���������[��֪2SO2(g)��O2(g) ![]() 2SO3(g)����H����196.6 kJ��mol��1]��
2SO3(g)����H����196.6 kJ��mol��1]��
���� | �� | �� | �� | �� |
��ʼͶ���� | 2 mol SO2��1 mol O2 | 1 mol SO2��0.5 mol O2 | 2 mol SO3 | 2 mol SO2��2 mol O2 |
��Ӧ�ų������յ�����(kJ) | a | b | c | d |
ƽ��ʱ c(SO3) (mol��L��1) | e | f | g | h |
���й�ϵ��ȷ����(����)
A. a��c��e��g B. a��2b��e��2f
C. a��d��e��h D. c��98.3e��196.6