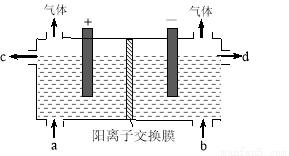
��Ŀ����
���⻯��(NaBH4)Ϊ��ɫ��ĩ��������ˮ���⣬�����������(�۵㣺-101�棬�е㣺33��)���ڸɿ������ȶ�����ʪ�����зֽ⣬�����ϳɺ��л��ϳ��г��õ�ѡ���Ի�ԭ����ij�о�С�����ƫ������(NaBO2)Ϊ��Ҫԭ���Ʊ�NaBH4�����������£�
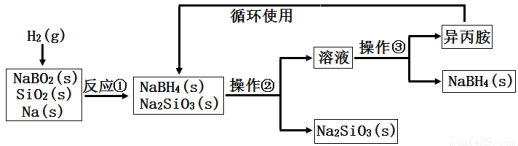
����˵������ ȷ����
ȷ����
A��ʵ������ȡ����������Ҫ�õ���ʵ����Ʒ�����ӡ���ֽ������Ƭ��С��
B�������ڡ������۷ֱ��ǹ����������ᾧ
C����Ӧ�ټ���֮ǰ�轫��Ӧ��������100�����ϲ�ͨ�����
D����Ӧ�����������뻹ԭ�������ʵ���֮��Ϊ1��2
��ϰ��ϵ�д�
 ��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ��ټ��ִ�ѧ������ϵ�д� �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д�
�����Ŀ


 CH3COO����H����Ҫʹ��Һ��c(H��)/c(CH3COOH)ֵ�����Բ�ȡ�Ĵ�ʩ�� �� ��
CH3COO����H����Ҫʹ��Һ��c(H��)/c(CH3COOH)ֵ�����Բ�ȡ�Ĵ�ʩ�� �� ��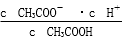 ��ֵ________(���������С�����䡱)��
��ֵ________(���������С�����䡱)�� ��1H2A��Һϡ�͵�20������Һ��pH��__________��
��1H2A��Һϡ�͵�20������Һ��pH��__________�� ��
�� ��һ���� D����ȷ��
��һ���� D����ȷ��
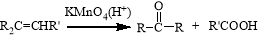 (R��R����������)
(R��R����������)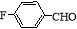 ������ ��
������ ��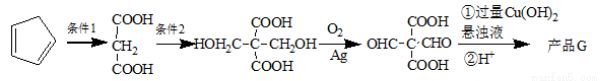
 ��
��