��Ŀ����
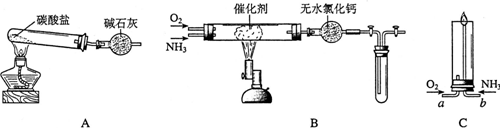
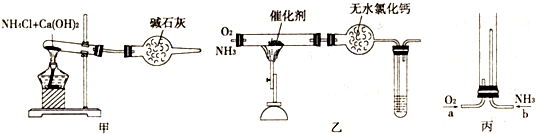
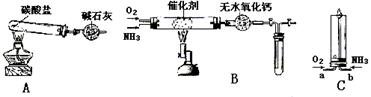
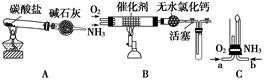
������ʾ�����������ڴ����а���ȼ�ա�����ijУ��ѧС��ѧ�������ͼ6-14װ��(ͼ�����еȼг�װ������ȥ)���а����������ڲ�ͬ�����·�Ӧ��ʵ�顣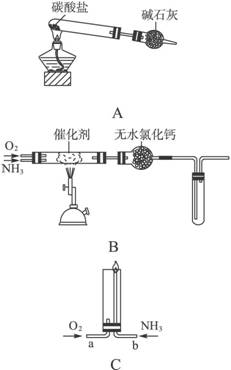
ͼ6-14
(1)��װ��A��ȡ����������İ��������Թ���̼���εĻ�ѧʽ��___________����ʯ�ҵ�����____________________��
(2)�������İ��������������ͨ��װ��B (����Ϊ��ʯ��)�У��þƾ���Ƽ��ȣ�
�ٰ��������Ļ�ѧ����ʽ��__________�Թ��������Ϊ����ɫ���÷�Ӧ�Ļ�ѧ����ʽ��_________________��
��ֹͣ��Ӧ�������ر�B������������һ��ʱ����Թܽ����ˮ�У��Թ���������ɫ��dz�����ϻ�ѧ����ʽ˵��ԭ��____________________________________________��
(3)��������������A�����İ����ֱ��a��b���ܽ�����ͨ�뵽װ��C�У�����b���϶˵�ȼ������
��������ͨ����Ⱥ�˳����___________����������______________________________��
�ڰ���ȼ�յĻ�ѧ����ʽ��_____________________________��
������(1)��ͼʾ��Ϣ����̼�������ȷֽ⣬����ͨ����ʯ���ư��������ж�A��(NH4)2CO3��NH4HCO3����ʯ��������CO2��H2O�ġ�
(2)��Ҫ��д���������Ļ�ѧ����ʽ�����ǽ̲��е����ݡ�����NO2���ܱ������з������淴Ӧ2NO2![]() N2O4��������������N2O4�����ɣ�����ɫ��NO2�������ɫ��N2O4������ɫ��dz��
N2O4��������������N2O4�����ɣ�����ɫ��NO2�������ɫ��N2O4������ɫ��dz��
(3)��NH3�ڴ�����ȼ�����⡣NH3�ڿ������Dz���ȼ�յġ����װ�ã����NH3��O2���ܶȣ��Ϳ��Եó��𰸡�NH3�ڴ�����ȼ�գ�û�д�����Ҳû�зŵ���������ʱ��NH3�봿����Ӧֻ������N2��H2O��
�𰸣�(1)(NH4)2CO3(��NH4HCO3) ����CO2��H2O
(2)��4NH3+5O2![]() 4NO+6H2O
4NO+6H2O
2NO+O2====2NO2
��2NO2(g)![]() N2O4(g)����H��0�������¶�ʹƽ�������ƶ������ֺ���ɫNO2ת��Ϊ��ɫN2O4
N2O4(g)����H��0�������¶�ʹƽ�������ƶ������ֺ���ɫNO2ת��Ϊ��ɫN2O4
(3)����ͨ��O2����ͨ��NH3 ����ͨ�����������ڿ����в��ܵ�ȼ���ݳ������Ⱦ
��4NH3+3O2![]() 2N2+6H2O
2N2+6H2O

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�