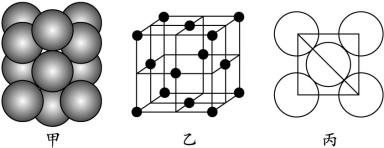
��Ŀ����
(1)�������ʾ�Ϊ1 mol������±����ش��й����⡣
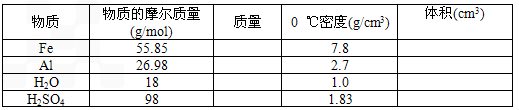
(3)�������������С��Ҫ���������أ�
�ٹ������ʵ�����Ŀ�������Ĵ�С������֮��ľ��롣���Թ�̬��Һ̬������˵���������ǵ�����ľ����Ǻ�С�ģ����1 mol��̬��Һ̬�������С��Ҫ������________(����ţ���ͬ)������̬������˵����̬����֮��ľ����Ƿ���ֱ����10�����ң����1 mol��̬���ʵ������С��Ҫ������________��
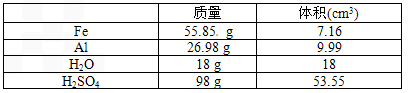
(3)�ڣ���

 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�[���ʽṹ������] ��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⡣
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ���: I1=738kJ/mol I2 = 1451 kJ/mol I3 = 7733kJ/mol I4 = 10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵ����� |
��1�� ��֪BA5 Ϊ���ӻ����д�������ʽ
��2�� B��̬ԭ����������ߵĵ��ӣ���������ڿռ��� ������ԭ�ӹ���� ��
��3�� ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�Ϊ��![]()
��ͬѧ�����ĵ����Ų�ͼΥ����
��4�� Gλ�� �� �����۵����Ų�ʽΪ
��5�� DE3 ����ԭ�ӵ��ӻ���ʽΪ ���ü۲���ӶԻ��������Ʋ���ռ乹��Ϊ
��6�� ����FԪ�صķ����� ������ ԭ�ӽṹ��֪ʶ���Ͳ����������ԭ����
ԭ�ӽṹ��֪ʶ���Ͳ����������ԭ����
��7�� FԪ�صľ�������ͼ��ʾ������þ������ܶ�Ϊa g/cm3�������ӵ�����ΪNA��Fԭ�ӵ�Ħ������ΪM����Fԭ�ӵİ뾶Ϊ cm
����ѧ��ѡ��3�����ʽṹ�����ʡ���15�֣�
��������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⡣
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ��ԭ�Ӱ뾶��С��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ���: I1=738kJ/mol I2 = 1451 kJ/mol I3 = 7733kJ/mol I4 = 10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵ����� |
��1����֪BA5Ϊ���ӻ����д�������ʽ
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ��� ������ԭ�ӹ���� ��
��3��ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�Ϊ��
��ͬѧ�����ĵ����Ų�ͼΥ����
��4��Gλ�� �� �����۵����Ų�ʽΪ
��5��DE3 ����ԭ�ӵ��ӻ���ʽΪ ���ü۲���ӶԻ��������Ʋ���ռ乹��Ϊ
��6������FԪ�صķ����� ������ԭ�ӽṹ��֪ʶ���Ͳ����������ԭ����
��7��FԪ�صľ�������ͼ��ʾ������þ������ܶ�Ϊa g/cm3�������ӵ�����ΪNA��
Fԭ�ӵ�Ħ������ΪM����Fԭ�ӵİ뾶Ϊ cm
��������Ԫ�أ�����A��B��C��DΪ����������Ԫ�أ�E��FΪ��������Ԫ�أ����ǵ�ԭ����������������������������Ϣ���ش����⡣
| AԪ��ԭ�ӵĺ���p����������s����������1 |
| BԪ��ԭ�Ӻ���s����������p����������ȣ��Ҳ���AԪ����ͬһ���� |
| Cԭ�Ӻ�������p���ȫ������� |
| DԪ�ص������������������IJ�Ϊ4 |
| E��ǰ�������е縺����С��Ԫ�� |
| F�����ڱ��ĵ����� |
��ijͬѧ����������Ϣ��������B�����Ų�ͼ��ͼ
 ��Υ���� ԭ����
��Υ���� ԭ������Fλ�� �� �������̬ԭ���� ���˶�״̬��
��CD3 ����ԭ�ӵ��ӻ���ʽΪ ���ü۲���ӶԻ��������Ʋ�����ӿռ乹��Ϊ ������EԪ�صķ����� ��
����ij�������ʾ�����ԭ�ӵĶѻ���ʽ����ͼ����ʾ���侧����������ͼ����ʾ��ԭ��֮���λ�ù�ϵ��ƽ��ͼ����ͼ����ʾ�����и�ԭ�ӵ���λ��Ϊ ���õ��ʾ�����ԭ�ӵĶѻ���ʽΪ���ֻ����ѻ���ʽ�е� ������֪�ý�����ԭ�Ӱ뾶Ϊd cm��NA���������ӵ����������������ԭ������ΪM����þ�����ܶ�Ϊ______g��cm��3(����ĸ��ʾ)��