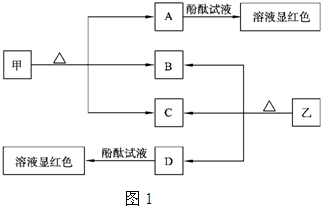
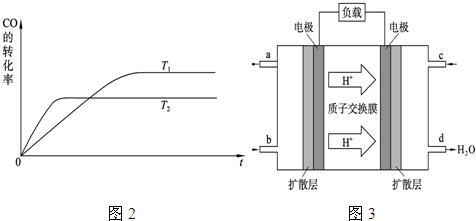
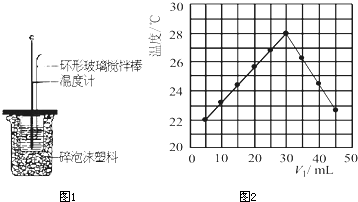
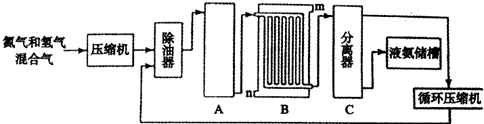
��Ŀ����
(1)1.2 mol��L-1��ij����������Zn+����Һ10 mL�����ð�30 mL���ʵ���Ũ��Ϊ0.4 mol��L-1��ij���е�(2)20 mL 1 mol��L-1��NaCl��40 mL 0.5 mol��L-1 CaCl2��Ϻ����Һ�У�Cl-�����ʵ���Ũ����_________��
(3)�ڱ�״���£�700 L NH3�����ʵ���Ϊ_________��ȫ���ܽ���1 Lˮ�У�������Һ����������Ϊ_________������ð�ˮ���ܶ�Ϊ0.85 g��cm-3����ˮ�����Ϊ_________ L�����ʵ���Ũ��Ϊ_________��
(4)��֪��������Ϊ37%��Ũ������ܶ�Ϊ1.19 g��cm-3������������ʵ���Ũ��Ϊ_________������200 mL 0.5 mol��L-1������Һ��Ҫ��Ũ����_________mL��
(5)�����£���Է�������ΪM��ij��ˮ��A���ܽ��ΪS g������ʱ�����α�����Һ����������Ϊ_________�������֪�ñ�����Һ���ܶ�Ϊ�� g��cm-3�������Һ�����ʵ���Ũ��Ϊ_________��
(1)2 (2)1 mol��L-1
(3)31.25 mol 34.7% 1.8 17.36 mol��L-1
(4)12.1 mol��L-1 8.3
(5)![]() ��100%
��100% ![]() mol��L-1
mol��L-1

 ��ְٷְټ���ϵ�д�
��ְٷְټ���ϵ�д� �����ƻ���ĩ��̶�100��ϵ�д�
�����ƻ���ĩ��̶�100��ϵ�д� �ܿ���ȫ��100��ϵ�д�
�ܿ���ȫ��100��ϵ�д�
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||