��Ŀ����
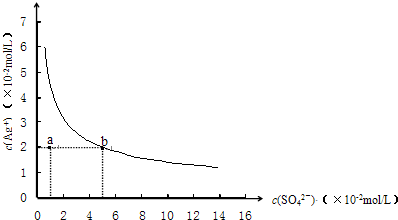
ij�¶�ʱ��Ag2SO4��ˮ��Һ�еij����ܽ�ƽ��������ͼ��ʾ��
��1��a���ʾAg2SO4______ ������͡������͡��� ��Һ��
��2��b���Ӧ��Ksp______d���Ӧ��Ksp�����������������=������
��3���ֽ�������Ag2SO4�ֱ���룺
a.40mL0.01mol?L-1 K2SO4��Һ��b.10mL����ˮ��
c.10mL0.02mol?L-1H2SO4��Һ��
��Ag2SO4���ܽ���ɴ�С������˳��Ϊ______������ĸ��
��4�������й�Ag2SO4˵����ȷ����______
A�����д���SO42-����Һ�п϶�������Ag+
B��Ag2SO4���ܶȻ�������Ksp��Ϊ1.6��10-5 ��mol?L-1��3
C��0.02mol?L-1��AgNO3��Һ��0.2mol?L-1��Na2SO4��Һ����� ��ϲ������ɳ���
��5����Ag2SO4��Һ�м���������Na2CrO4���壬�ɹ۲쵽ש��ɫ������Ag2CrO4Ϊש��ɫ����д������ת�������ӷ���ʽ______��
��1��a���ʾAg2SO4______ ������͡������͡��� ��Һ��
��2��b���Ӧ��Ksp______d���Ӧ��Ksp�����������������=������
��3���ֽ�������Ag2SO4�ֱ���룺
a.40mL0.01mol?L-1 K2SO4��Һ��b.10mL����ˮ��
c.10mL0.02mol?L-1H2SO4��Һ��
��Ag2SO4���ܽ���ɴ�С������˳��Ϊ______������ĸ��
��4�������й�Ag2SO4˵����ȷ����______
A�����д���SO42-����Һ�п϶�������Ag+
B��Ag2SO4���ܶȻ�������Ksp��Ϊ1.6��10-5 ��mol?L-1��3
C��0.02mol?L-1��AgNO3��Һ��0.2mol?L-1��Na2SO4��Һ����� ��ϲ������ɳ���
��5����Ag2SO4��Һ�м���������Na2CrO4���壬�ɹ۲쵽ש��ɫ������Ag2CrO4Ϊש��ɫ����д������ת�������ӷ���ʽ______��

��1������ͼ�����һ���¶��µ��ܶȻ�Ksp=c2��Ag+����c��SO42-��=��2��10-2��2��4��10-2=1.6��10-5��mol?L-1��3��ͼ����a�㴦������Ũ��Ϊ��c��SO42-��=1��10-2mol/L��c��Ag+��=2��10-2mol/L��c2��Ag+����c��SO42-��=4��10-6��ksp�����DZ�����Һ���ʴ�Ϊ�������ͣ�
��2��b��d���㴦�������ϵĵ�Ϊ����״̬����Һ�д����ܽ����ƽ�⣬�ܶȻ��������¶ȱ仯������Ũ�ȱ仯��Ksp���䣬�ʴ�Ϊ��=��
��3��һ���¶��µ��ܶȻ�Ksp=c2��Ag+����c��SO42-��=��2��10-2��2��4��10-2=1.6��10-5��mol?L-1��3����������Ag2SO4�ֱ���룺a.40mL0.01mol?L-1 K2SO4��Һ��0.01mol/L��������������������ܽ����ƽ�⣻b.10mL����ˮ���γɱ�����Һ��c.10mL0.02mol?L-1H2SO4��Һ�У�0.02mol/L��������������Ƴ����ܽ�ƽ�⣻��Ag2SO4���ܽ���ɴ�С������˳��Ϊ��b��a��c���ʴ�Ϊ��b��a��c��
��4��A����������Һ�д��ڳ����ܽ�ƽ�⣬�����Ӵ��ڣ���A����
B������ͼ�����һ���¶��µ��ܶȻ�Ksp=c2��Ag+����c��SO42-��=��2��10-2��2��4��10-2=1.6��10-5��mol?L-1��3����B��ȷ��
C��0.02mol?L-1��AgNO3��Һ��0.2mol?L-1��Na2SO4��Һ�������ϣ���Һ��C��Ag+��=0.01mol/L��C��SO42-��=0.1mol/L��c2��Ag+����c��SO42-��=10-5��ksp�����DZ�����Һ���������ɣ���C��ȷ��
�ʴ�Ϊ��BC��
��5����Ag2SO4��Һ�м���������Na2CrO4���壬�ɹ۲쵽ש��ɫ������Ag2CrO4Ϊש��ɫ����˵��Ag2CrO4�ܽ���С��Ag2SO4�����˳���ת������Ӧ�����ӷ���ʽΪ��Ag2SO4+CrO42-?Ag2CrO4+SO42-���ʴ�Ϊ��Ag2SO4+CrO42-?Ag2CrO4+SO42-��
��2��b��d���㴦�������ϵĵ�Ϊ����״̬����Һ�д����ܽ����ƽ�⣬�ܶȻ��������¶ȱ仯������Ũ�ȱ仯��Ksp���䣬�ʴ�Ϊ��=��
��3��һ���¶��µ��ܶȻ�Ksp=c2��Ag+����c��SO42-��=��2��10-2��2��4��10-2=1.6��10-5��mol?L-1��3����������Ag2SO4�ֱ���룺a.40mL0.01mol?L-1 K2SO4��Һ��0.01mol/L��������������������ܽ����ƽ�⣻b.10mL����ˮ���γɱ�����Һ��c.10mL0.02mol?L-1H2SO4��Һ�У�0.02mol/L��������������Ƴ����ܽ�ƽ�⣻��Ag2SO4���ܽ���ɴ�С������˳��Ϊ��b��a��c���ʴ�Ϊ��b��a��c��
��4��A����������Һ�д��ڳ����ܽ�ƽ�⣬�����Ӵ��ڣ���A����
B������ͼ�����һ���¶��µ��ܶȻ�Ksp=c2��Ag+����c��SO42-��=��2��10-2��2��4��10-2=1.6��10-5��mol?L-1��3����B��ȷ��
C��0.02mol?L-1��AgNO3��Һ��0.2mol?L-1��Na2SO4��Һ�������ϣ���Һ��C��Ag+��=0.01mol/L��C��SO42-��=0.1mol/L��c2��Ag+����c��SO42-��=10-5��ksp�����DZ�����Һ���������ɣ���C��ȷ��
�ʴ�Ϊ��BC��
��5����Ag2SO4��Һ�м���������Na2CrO4���壬�ɹ۲쵽ש��ɫ������Ag2CrO4Ϊש��ɫ����˵��Ag2CrO4�ܽ���С��Ag2SO4�����˳���ת������Ӧ�����ӷ���ʽΪ��Ag2SO4+CrO42-?Ag2CrO4+SO42-���ʴ�Ϊ��Ag2SO4+CrO42-?Ag2CrO4+SO42-��

��ϰ��ϵ�д�
 Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д� Сѧͬ�����������ܾ�ϵ�д�
Сѧͬ�����������ܾ�ϵ�д�
�����Ŀ


 ij�¶�ʱ��Ag2SO4��ˮ��Һ�еij����ܽ�ƽ��������ͼ��ʾ��
ij�¶�ʱ��Ag2SO4��ˮ��Һ�еij����ܽ�ƽ��������ͼ��ʾ�� ij�¶�ʱ��Ag2SO4��ˮ��Һ�еij����ܽ�ƽ��������ͼ��ʾ������˵���в���ȷ���ǣ�������
ij�¶�ʱ��Ag2SO4��ˮ��Һ�еij����ܽ�ƽ��������ͼ��ʾ������˵���в���ȷ���ǣ�������