��Ŀ����
����Ŀ����̽���һ��Ӧ�ù㷺�Ĺ������ϣ���ϳ�·������(�����Լ��Ͳ�����ȥ)��

��֪��I. 
II. 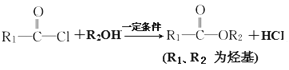
��1���ǵ�����________
��2����֪���ܷ���ˮ�ⷴӦ����X�Ľṹ��ʽ________
��3��C��D�ķ�Ӧ������_______
��4����̽��Ľṹ��ʽ_______________________
��5��B��C�ڢٲ���Ӧ�Ļ�ѧ����ʽΪ_____________________
��6������õķ���ʽ��ͬ���������������ģȵĽṹ��_______�֡�
�ٷ��ӽṹ��ֻ��һ���ǻ�������FeCl3��Һ������ɫ��Ӧ
�ڲ��ܷ���������Ӧ
�۷����г���������������״�ṹ
��7�����������Ϣд���ԣ�Ϊԭ���Ʊ��������ĺϳ�·������ͼ(�����Լ���ѡ)��______________
�ϳ�·������ͼʾ�����£�CH3CHO![]() CH3COOH
CH3COOH ![]() CH3COOCH2CH3
CH3COOCH2CH3
���𰸡� ����ϩ�� CH3COOH ȡ����Ӧ ![]()
![]() 3
3 
����������D�Ľṹ��֪��A�뱽��ȩ��Ӧ�õ�B�������ʵķ���ʽ����Ϣ����֪AΪ��ȩ��BΪ![]() ��B��������Һ����������Ӧ���ữ�õ�C����CΪ
��B��������Һ����������Ӧ���ữ�õ�C����CΪ![]() ����Ȳ��X�ӳ�����E��E������Ϸ�Ӧ�õ�F��Fһ����������G������G�Ľṹ�����E�Ļ�ѧʽ��˵��Fˮ������G�����ᣬ��E�����к���C=C˫������Ϸ���ʽ��֪��XΪCH3COOH����EΪCH3COOCH=CH2��FΪ
����Ȳ��X�ӳ�����E��E������Ϸ�Ӧ�õ�F��Fһ����������G������G�Ľṹ�����E�Ļ�ѧʽ��˵��Fˮ������G�����ᣬ��E�����к���C=C˫������Ϸ���ʽ��֪��XΪCH3COOH����EΪCH3COOCH=CH2��FΪ![]() ��G��D������Ϣ��ķ�Ӧ�õ���̽������̽��Ľṹ��ʽΪ��
��G��D������Ϣ��ķ�Ӧ�õ���̽������̽��Ľṹ��ʽΪ��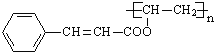 ��
��
��1����Ϊ![]() ������Ϊ����ϩ�����ʴ�Ϊ������ϩ����
������Ϊ����ϩ�����ʴ�Ϊ������ϩ����
��2����������������XΪCH3COOH���ʴ�Ϊ��CH3COOH��
��3��DΪ![]() �����ݽṹ��IJ���֪��C��D�ķ�Ӧ������ȡ����Ӧ���ʴ�Ϊ��ȡ����Ӧ��
�����ݽṹ��IJ���֪��C��D�ķ�Ӧ������ȡ����Ӧ���ʴ�Ϊ��ȡ����Ӧ��
��4������������������̽��Ľṹ��ʽΪ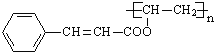 ���ʴ�Ϊ��
���ʴ�Ϊ��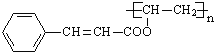 ��
��
��5��BΪ![]() ��B��������Һ����������Ӧ����Ӧ�Ļ�ѧ����ʽΪ
��B��������Һ����������Ӧ����Ӧ�Ļ�ѧ����ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
��6��CΪ![]() ������õķ���ʽ��ͬ���ٷ��ӽṹ��ֻ��һ���ǻ�������FeCl3��Һ������ɫ��Ӧ��˵���Ƿ��ǻ����ڲ��ܷ���������Ӧ��˵��������ȩ�����۷����г���������������״�ṹ��˵�������Ϻ����ǻ���-COCH=CH2�����������ģȵĽṹ��3�ֽṹ����λ����λ����λ�����ʴ�Ϊ��3��
������õķ���ʽ��ͬ���ٷ��ӽṹ��ֻ��һ���ǻ�������FeCl3��Һ������ɫ��Ӧ��˵���Ƿ��ǻ����ڲ��ܷ���������Ӧ��˵��������ȩ�����۷����г���������������״�ṹ��˵�������Ϻ����ǻ���-COCH=CH2�����������ģȵĽṹ��3�ֽṹ����λ����λ����λ�����ʴ�Ϊ��3��
��7����CH3CHOΪԭ���Ʊ���������������������ȩ�ͼ�ȩ��ϡNaOH��Һ�з�Ӧ����CH2=CHCHO��CH2=CHCHO��Br2�����ӳɷ�Ӧ����CH2BrCHBrCHO��CH2BrCHBrCHO��NaOHˮ��Һ�ڼ��������·���ȡ����Ӧ����CH2��OH��CH��OH��CHO������������ӳɼ��ɣ��ϳ�·��Ϊ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�