��Ŀ����
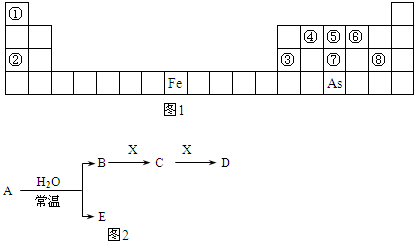
ij��������X�������й�ϵͼ������A��B�ֱ���X�������ۡ������۽������ӣ���ش�
��1��д��X������ ��Y�Ļ�ѧ ʽ ��
ʽ ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
�� X��ϡ���ᷴӦ����A��ij����������ӷ���ʽ
�� +2�۵�A��Y������Ӧ����+3�۵�B�����ӷ���ʽ
�� X��ĩ������ͭ��Һ�����û���Ӧ�����ӷ���ʽ

�� X�������������X2O3�������۷�Ӧ�Ļ�ѧ����ʽ
��3��A��Һ��NaOH��Һ�ڿ����з�Ӧ������  ��
��
��д���йصĻ�ѧ����ʽ ��
��1���� Cl2
��2��Fe + 2H+ =" " Fe2+ + H2#
2Fe2+ + Cl2 = 2Fe3+ + 2Cl-
Fe + Cu2+ = Fe2+ + Cu
Al + Fe2O3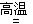 Al2O3 + Fe
Al2O3 + Fe
��3���Ȳ�����ɫ������Ȼ��ת��ɻ���ɫ������ɺ��ɫ
4Fe(OH)2 + O2 + 2H2O = 4Fe(OH)3
����

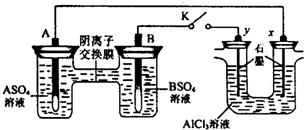
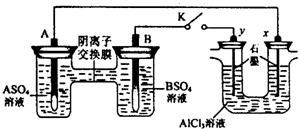
| A����Һ��c��A2+��Ũ�ȼ�С | B��B�ĵ缫��Ӧ��B-2e-?B2+ | C��y�缫����H2������������ԭ��Ӧ | D����Ӧ���ڣ�x�缫��Χ���ְ�ɫ��״���������ó����ܽ� |