��Ŀ����
ij����Һ����Ҫ�ɷ�ΪNaCl��NaClO���ڿ�����������CO2�����ʣ���NaCl��NaClO�����������¿ɷ�����Ӧ��ClO- + Cl- + 2H+ = Cl2��+ H2O��ijѧϰС����̽��������Һ�ı��������
��1��ȡ��������Һ�����Թ��У���������һ��Ũ�ȵ����ᣬ������ų���ͨ������װ�ü�������ijɷֿ����ж�����Һ�Ƿ���ʡ�
ѧϰС���о�����Ϊ����������������֣��ף����ֱ��ʣ��ң�δ���ʣ����� ��
Ϊ����֤����Ϊ�ף����������ʵ�鷽������ѡ�Լ���
��98%��Ũ���� ��1%��Ʒ����Һ ��1.0 mol��L-1��KI��������Һ ��1.0 mol��L-1 ��NaOH��Һ �ݳ���ʯ��ˮ �ޱ���NaCl��Һ
| �����Լ� | Ԥ������ͽ��� |
| �Թ�A�м����� ������ţ��� �Թ�B�м�1%Ʒ����Һ�� �Թ�C�м� ������ţ��� | ��___ ___�� ��׳����� |
��2���õζ����ⶨ����Һ��NaClO��Ũ�ȣ��ζ������漰�ķ�Ӧ�У�NaClO + Na2SO3 = NaCl+ Na2SO4 ��2KMnO4 + 5Na2SO3+ 3H2SO4 = K2SO4 + 2MnSO4 + 5Na2SO4 + 3H2O����ʵ�鲽�����£�
����ȡ25.00 mL����Һ������ƿ�У����������a mol��L-1 Na2SO3��Һv1 mL��
����ʹ�õζ���֮ǰ���Ƚ��еIJ�����____����b mol��L-1������KMnO4��Һװ�� �У��ζ���KMnO4��ʣ���Na2SO3������Ӧ������Һ����ɫ���dz��ɫ���ұ��ְ�����ں�ɫ����ʱ��ֹͣ�ζ�����¼���ݡ�
���ظ��ζ�����2�Σ�ƽ����������KMnO4��Һv2 mL��������Һ��NaClO��Ũ��Ϊ mol��L-1���ú�a��b��v1��v2�Ĵ���ʽ��ʾ����
��15�֣���1��ȫ�����ʣ�2�֣����ۣ�2�֣�
�ݣ�2�֣�A����Һ����ɫ��B����Һ����ɫ��
C����Һ����ǣ�2�֣�
��2�����ζ����Ƿ�©Һ�����©����2�֣�������ʽ��2�֣����ۣ�av1-5/2bv2����1/25 (3��)
���������������1������������������֣��ף����ֱ��ʣ��ң�δ���ʣ���˵������������Ӧ����ȫ�����ʡ�
��2��������֪����Ϣ��֪��Ҫ�����Ƿ���ʣ�����Ҫ��������Һ�����ᷴӦ���ɵ��������Ƿ���������������̼���ݴ˿����ж�����Һ�ı��������װ��A�����Ǽ�����������1.0mol/L��KI������Һ��װ��B�����Ǽ��������Ƿ������װ��C���������̼���ù�������ʯ��ˮ���ɡ���A����Һ����ɫ��B����Һ����ɫ��C����Һ����ǣ�������Һ���ֱ��ʣ���A����Һ����ɫ��B����Һ����ɫ���ޱ仯����C����Һ������ǣ��ޱ仯����������Һδ���ʣ���A����Һ������ɫ���ޱ仯����B����Һ����ɫ���ޱ仯����C����Һ�����������Һ��ȫ���ʡ�
��3������ʹ�õζ���֮ǰ���Ƚ��еIJ����Ǽ��ζ����Ƿ�©Һ�����©��������KMnO4��Һ����ǿ�����ԣ����Ը�ʴ��Ƥ�ܣ�Ӧװ����ʽ�ζ����С�
��25.00mL����Һ��Ҫ����KMnO4��Һ���Ϊv2mL��KMnO4�����ʵ���Ϊv2��10-3L��bmol/L��v2��b��10-3mol�����ݷ�Ӧ�ķ���ʽ�ɵó���ϵʽ5Na2SO3��2KMnO4���ݴ˿�֪25.00mL��Һδ��Ӧn(Na2SO3)��2.5��v2��b��10-3mol��25mL��Һ�м�����ܵ�n(Na2SO3)��v1��10-3L��a mol/L��v1��a��10-3mol���μӷ�Ӧ����������Ϊv1��a��10-3mol��2.5��v2��b��10-3mol����v1a��2.5 v2b����10-3mol�����ݹ�ϵʽNaClO��Na2SO3��֪��25mL��Һ��n��NaClO������v1a��2.5 v2b����10-3mol����������Һ��NaClO��Ũ��Ϊ��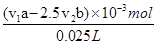 ����av1��5/2bv2����1/25��
����av1��5/2bv2����1/25��
���㣺�������ʵļ��顢������ѡ��ʵ�鷽������������Լ����ʵ���Ũ�ȵ��йؼ����

����ʵ��װ�ã����̶ֹ�װ����ȥ�����й�������ȷ����
| A��ͼ1���Խ�������к͵ζ�ʵ�� |
| B��ͼ2���Խ����к��ȵIJⶨʵ�� |
| C��ͼ3����֤�¶ȶԻ�ѧƽ���Ӱ�� |
| D��ͼ4�ɼ���ʳ��ˮͨ���IJ��ֲ��� |
����ʵ�鷽���ͽ��ͻ���۶���ȷ����
| | ʵ��Ŀ�� | ʵ�鷽�� | ���ͻ���� |
| A | ����CH2=CH-CHO�к�̼̼˫�� | ����ϩȩ��Һ������ˮ�У���ˮ��ɫ | ��ϩȩ��̼̼˫�����嵥�ʷ����˼ӳɷ�Ӧ |
| B | ȷ��ij����Ũ��Һ������ | ��պ��Ũ��ˮ�IJ����������Լ�ƿ�ڣ��д������� | ������һ��Ϊ���� |
| C | ����һ�ݺ���ɫ����ɷ� | ʪ��ĵ��۵⻯����ֽ���������У���ֽ���� | ������һ��ΪBr2 |
| D | ̽����֬���������ˮ������� | ��֬����������м���NaOH��Һ���Ⱥ��ٷֲ� | ��֬�������������������Ӧ |
���л�ѧʵ�������ܹ��ﵽĿ�ĵ���
| A��Ϊ����KCl��AlCl3��MgCl2��Һ���ֱ���������Һ�еμ�NaOH��Һ������ |
| B������ȥ��������Һ�е�NaCl���ֲ��ı������ʣ��ɼ�������BaCl2��Һ����� |
| C������ˮ��pH�����ò�����պȡ��ˮ����pH��ֽ�ϣ������ɫ��ͱ���ɫ���Ƚ� |
| D��Ϊ��֤����¯���к����������ɽ���¯��ͨ�����ȵ�����ͭ��ĩ������ɫ��ĩ�Ƿ���ɫ |


 H2O + CH3CH2��O��CH2CH3 (����)
H2O + CH3CH2��O��CH2CH3 (����)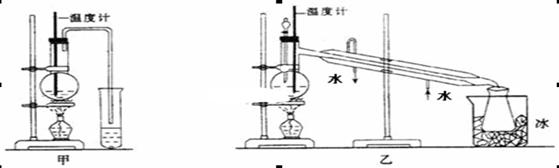
 NH2COONH4(s) ��H��0
NH2COONH4(s) ��H��0