��Ŀ����
ij��ѧ��ȤС��ͬѧչ����Ư����������(NaClO2)���о���
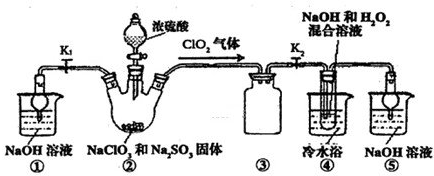
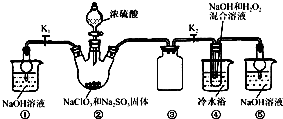
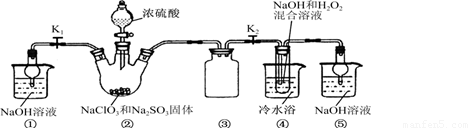
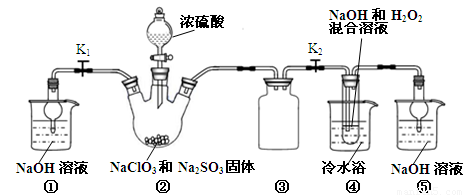
ʵ��I����ȡNaClO2����
��֪��NaClO2������Һ���¶ȵ���38��ʱ����Ʒ����NaClO2��3H2O������38��ʱ����������NaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl��������ͼ��ʾװ�ý���ʵ�顣
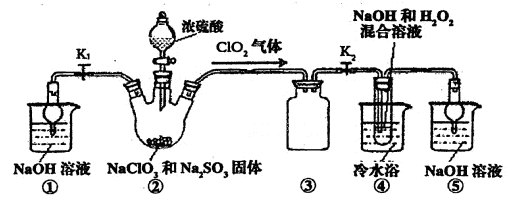
��1��װ�â۵�������
װ�âٵ�������
��2��װ�â��в���ClO2�Ļ�ԭ����
װ�â����Ʊ�ClO2�Ļ�ѧ����ʽΪ
��3����װ�âܷ�Ӧ�����Һ���NaClO2����IJ�������Ϊ��
�ټ�ѹ��55�������ᾧ���ڳ��ȹ��ˣ��� ���ܵ���60�����õ���Ʒ��
ʵ��ⶨij����������Ʒ�Ĵ��ȡ�
�������ʵ�鷽����������ʵ�飺
��ȷ��ȡ��������������ƷС���ձ��У�������������ˮ�����ĵ⻯�ؾ��壬�ٵ���������ϡ���ᣬ��ַ�Ӧ(��֪��ClO2-+4I-+4H+=2H2O+2I2+Cl-)�������û��Һ���250mL������Һ��
����ȡ25��00mL������Һ����ƿ�У��Ӽ��ε�����Һ����c mol��L-1 Na2S2O3��Һ�ζ������ζ��յ㡣�ظ�2�Σ����ƽ��ֵΪV mL(��֪��I2+2S2O32-=2I-+S4O62-)��
��4���ﵽ�ζ��յ�ʱ������Ϊ
��5������Ʒ��NaClO2����������Ϊ (�ú�m��c��V�Ĵ���ʽ��ʾ)��
��6���ڵζ�������ȷ���������£���ʵ���ý��ƫ�ߣ�ԭ�������ӷ���ʽ��ʾΪ ��
��1����ֹ������2�֣�������װ����ĩ��Ӧ��ClO2,��ֹ������Ⱦ������2�֣�
��2��Na2SO3��2�֣�;2NaOH��2ClO2��H2O2=2NaClO2��2H2O��O2����3�֣�
��3����38�桫60�����ˮϴ�ӣ�2�֣�
��4���μ����һ��Һ��ʱ��Һ����ɫ�����ɫ�Ұ�����ڲ���ɫ��2�֣�
��5��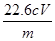 %��3�֣�
%��3�֣�
��6��4I�D��O2��4H�� =2I2��2H2O��2�֣�
��������
�����������1��װ�â������巴Ӧ��װ����ѹǿ���ͣ�װ�â۷�ֹ������װ������δ��Ӧ��ClO2��װ�âٿ�������δ��Ӧ��ClO2����ֹ�ݳ���Ⱦ������
��Ϊ����ֹ����������δ��Ӧ��ClO2����ֹ�ݳ���Ⱦ������
��2���������ƾ��л�ԭ�ԣ��ڷ�Ӧ������ԭ����
װ�âܷ�Ӧ�����Һ���NaClO2���壬��װ�â�������NaClO2��ClԪ�صĻ��ϼ۽��ͣ�˫��ˮӦ���ֻ�ԭ�ԣ����������ɣ����ԭ���غ��֪������ˮ���ɣ���ƽ��ʽΪ��2NaOH+2ClO2+H2O2=2NaClO2+2H2O+O2��
����Na2SO3��2NaOH+2ClO2+H2O2=2NaClO2+2H2O+O2��
��3������Һ����ȡ���壬һ����������ᾧ�����ˡ�ϴ�ӡ�����ķ�����Ϊ��ֹ��������NaClO2��3H2O��Ӧ���ȹ��ˣ�����Ŀ��Ϣ��֪��Ӧ�����¶�38�桫60�����ϴ�ӣ�����60����
��Ϊ����38�桫60����ˮϴ�ӣ�
��4���������۱���ɫ����Ӧ����ʱ���ⷴӦ��ȫ���μ����һ��Na2S2O3��Һʱ��Һ����ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ��˵������ζ��յ㣬
��Ϊ���μ����һ��Na2S2O3��Һʱ��Һ����ɫ��Ϊ��ɫ�Ұ�����ڲ���ɫ��˵������ζ��յ㣻
��5������Ʒ��NaClO2����������Ϊa����
NaClO2��2I2��4S2O32��
90.5g 4mol
mag
c mol��L-1��V��10-3L��
����90.5g��mag=4mol��c mol��L-1��V��10-3L��
���a= %��
%��
���� %
%
��6��ʵ���ý��ƫ�ߣ�˵���ζ����ĵ�Na2S2O3��Һ���ƫ�ߣ���Һ�е�ĺ���ƫ�ߣ�Ӧ�����ɵĵ����ӱ���������Ϊ�⣬ͬʱ����ˮ����Ӧ���ӷ���ʽΪ4I��+O2+4H��=2I2+2H2O��
����4I��+O2+4H��=2I2+2H2O��
���㣺�ȡ��塢�⼰�仯������ۺ�Ӧ�ã�̽�����ʵ���ɻ�������ʵĺ������Ʊ�ʵ�鷽�������

 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д�