��Ŀ����
(����ʽÿ��2��,����ÿ��1��,��21�֡�)������ԭ��Ӧ�����ӷ�Ӧ�Ǹ�һ��ѧ������Ҫ��ѧ��Ӧ����,��ϸ˼���ش���������:
��������һ����ˮ����ķ�Ӧ:
��2Na + 2H2O =" 2NaOH" + H2��
��2Na2O2 + 2H2O =" 4NaOH" + O2��
��Na2O+ H2O ="2NaOH"
��3Fe+4H2O Fe3O4+4H2
Fe3O4+4H2
��2H2O 2H2��+ O2��
2H2��+ O2��
��2F2 + 2H2O =" 4HF" + O2
��1�����в�����������ԭ��Ӧ���� �����ţ�
��2������Щ������ԭ��Ӧ��,ˮֻ���������ķ�Ӧ�� �����ţ�
ˮֻ����ԭ���ķ�Ӧ�� �����ţ�ˮ����������,������ԭ���� �����ţ�
ˮ�Ȳ���������,�ֲ�����ԭ���� �����ţ�
��3�������������,������ԭ��Ӧ��ʵ���ǣ� ��
��4������������ѧ֪ʶ����3NO2+H2O �� 2HNO3+NO�Ƿ�����������ԭ��Ӧ�� ����ǡ����ǡ���,����,�����Ӧ���������� ,��ԭ���� ��
����д�����з�Ӧ�����ӷ���ʽ:
�����Ȼ�����Һ��������:
�ڹ���������̼ͨ�����ʯ��ˮ:
�����Ȼ�����Һ�м���������NaOH��Һ:
����д�����з�Ӧ�Ļ�ѧ����ʽ:
�ٶ�����������������Ӧ:
������������������Һ��Ӧ:
��ͭƬ��ϡ����ķ�Ӧ:
��������һ����ˮ����ķ�Ӧ:
��2Na + 2H2O =" 2NaOH" + H2��
��2Na2O2 + 2H2O =" 4NaOH" + O2��
��Na2O+ H2O ="2NaOH"
��3Fe+4H2O
 Fe3O4+4H2
Fe3O4+4H2��2H2O
 2H2��+ O2��
2H2��+ O2�� ��2F2 + 2H2O =" 4HF" + O2
��1�����в�����������ԭ��Ӧ���� �����ţ�
��2������Щ������ԭ��Ӧ��,ˮֻ���������ķ�Ӧ�� �����ţ�
ˮֻ����ԭ���ķ�Ӧ�� �����ţ�ˮ����������,������ԭ���� �����ţ�
ˮ�Ȳ���������,�ֲ�����ԭ���� �����ţ�
��3�������������,������ԭ��Ӧ��ʵ���ǣ� ��
| A�������е�ԭ��������� | B����Ԫ�صĵ�ʧ |
| C�����ӵĵ�ʧ���õ��ӶԵ�ƫ�� | D�����ϼ۵ĸı� |
����д�����з�Ӧ�����ӷ���ʽ:
�����Ȼ�����Һ��������:
�ڹ���������̼ͨ�����ʯ��ˮ:
�����Ȼ�����Һ�м���������NaOH��Һ:
����д�����з�Ӧ�Ļ�ѧ����ʽ:
�ٶ�����������������Ӧ:
������������������Һ��Ӧ:
��ͭƬ��ϡ����ķ�Ӧ:
����1���� (1��) ��2���٢�;��;��;�� ��ѡ��1����1�֣���4�֣�
��3��C (1��) ��4���ǣ� NO2�� NO2����1�֣���3�֣�
����2Fe3+ + Fe = 3Fe2+ ;CO2 + OH- = HCO3- ; Al3+ + 4OH- = AlO2- + 2H2O
����2SO2 + O2 ��
�� 2SO3 ;Cl2 + 2NaOH =" NaCl" + NaClO + H2O;
2SO3 ;Cl2 + 2NaOH =" NaCl" + NaClO + H2O;
3Cu + 8HNO3(ϡ) = 3Cu(NO3)2 + 2NO��+ 4H2O
��3��C (1��) ��4���ǣ� NO2�� NO2����1�֣���3�֣�
����2Fe3+ + Fe = 3Fe2+ ;CO2 + OH- = HCO3- ; Al3+ + 4OH- = AlO2- + 2H2O
����2SO2 + O2
 ��
�� 2SO3 ;Cl2 + 2NaOH =" NaCl" + NaClO + H2O;
2SO3 ;Cl2 + 2NaOH =" NaCl" + NaClO + H2O;3Cu + 8HNO3(ϡ) = 3Cu(NO3)2 + 2NO��+ 4H2O
�������������1�����в�����������ԭ��Ӧ�ļ����ϼ�û�з����仯�ķ�Ӧ��ֻ�Тۣ�
��2��ˮֻ���������ķ�Ӧ��ˮ�е�H���ϼ۽��ͣ�����Ӧ����H2��ѡ�٢ܣ�
ˮֻ����ԭ���ķ�Ӧ��ˮ�е�O���ϼ����ߣ�������Ӧ����O2��ͬʱO2������Դ��ˮ��ֻ��ѡ�ޣ�ˮ����������,������ԭ��������м�������������������ѡ�ݣ���������ԭ��Ӧ������ˮ�е����������ϼ�û�б仯����ֻ���Ǣڣ�
��3�������������,������ԭ��Ӧ��ʵ���ǵ��ӵĵ�ʧ���õ��ӶԵ�ƫ�ƣ����ֳ������ǻ��ϼ۵ĸı䣻
��4��3NO2+H2O �� 2HNO3+NO��N�Ļ��ϼ۷����˱仯����������ԭ��Ӧ��NO2��N��2��N���ϼ����ߵ�+5��HNO3����1��N���͵�+2�۵�NO��������������ԭ������NO2��
����2Fe3+ + Fe = 3Fe2+ ;CO2 + OH- = HCO3- ; Al3+ + 4OH- = AlO2- + 2H2O
����2SO2 + O2
 ��
�� 2SO3 ;Cl2 + 2NaOH =" NaCl" + NaClO + H2O;
2SO3 ;Cl2 + 2NaOH =" NaCl" + NaClO + H2O;3Cu + 8HNO3(ϡ) = 3Cu(NO3)2 + 2NO��+ 4H2O
������ѧ�����ڷ���ʽ����дһֱ���ѵ㣬��ƽʱ��дʱҪ����˼�����ϼ۵ı仯����ס�������ʵ������Ի�ԭ�ԣ��������ڷ���ʽ����д�����°빦�������á�

��ϰ��ϵ�д�
�����Ŀ
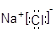
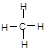


 Cl2�� + MnCl2 + 2H2O
Cl2�� + MnCl2 + 2H2O