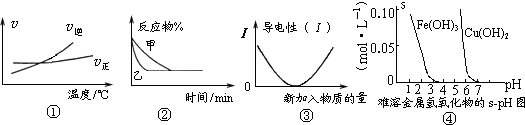
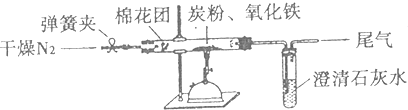
��Ŀ����
��2011?�Ϲ�����ģ���������ӷ���ʽ����ȷ���ǣ�������
������A����Ba��OH��2��Һ�еμ�NaHSO4��Һ��ǡ��Ϊ�����������ᱵ�������ơ�ˮ��
B��©д̼������������ķ�Ӧ��
C��ϡ�����������м��Ӧ��������������NO��ˮ��
D��KI��Һ��H2SO4�ữ��H2O2��Һ��Ϸ���������ԭ��Ӧ����ˮ���⡢����أ�
B��©д̼������������ķ�Ӧ��
C��ϡ�����������м��Ӧ��������������NO��ˮ��
D��KI��Һ��H2SO4�ữ��H2O2��Һ��Ϸ���������ԭ��Ӧ����ˮ���⡢����أ�
����⣺A����Ba��OH��2��Һ�еμ�NaHSO4��Һ��ǡ��Ϊ���Ե����ӷ�ӦΪBa2++2OH-+2H++SO42-=BaSO4��+2H2O����A����
B��NH4HCO3��Һ�����KOHŨ��Һ���ȵ����ӷ�ӦΪHCO3++NH4++2OH
NH3��+H2O+CO32-����B����
C��ϡ�����������м��Ӧ�����ӷ�ӦΪ3Fe+8H++2NO3-=3Fe2++2NO��+4H2O����C����
D��KI��Һ��H2SO4�ữ��H2O2��Һ��ϵ����ӷ�ӦΪ2I-+H2O2+2H+=2H2O+I2����D��ȷ��
��ѡD��
B��NH4HCO3��Һ�����KOHŨ��Һ���ȵ����ӷ�ӦΪHCO3++NH4++2OH
| ||
C��ϡ�����������м��Ӧ�����ӷ�ӦΪ3Fe+8H++2NO3-=3Fe2++2NO��+4H2O����C����
D��KI��Һ��H2SO4�ữ��H2O2��Һ��ϵ����ӷ�ӦΪ2I-+H2O2+2H+=2H2O+I2����D��ȷ��
��ѡD��
���������⿼�����ӷ�Ӧ����ʽ����д����ȷ�����Ļ�ѧ��Ӧ�ǽ����Ĺؼ�����ע�����ӷ�Ӧ����ʽ����д�����������Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ



 H++Cl-+HClO�������CaCO3��ĩ��H+��Ӧ��ƽ�������ƶ���HClOŨ������
H++Cl-+HClO�������CaCO3��ĩ��H+��Ӧ��ƽ�������ƶ���HClOŨ������