��Ŀ����
A��B��C��D��E����Ԫ�أ����ǵ�ԭ���������������Ҷ������ڱ���ǰ20��Ԫ�أ�Aԭ�ӻ�̬ʱ��p�ܼ���������δ�ɶԵ��ӣ�BԪ�ص�ԭ�ӻ�̬ʱ��s�ܼ���p�ܼ��ĵ�������ȣ�B��Cͬ���ڣ���C�DZ������е縺������Ԫ�أ�Dԭ�ӵĵ�һ�����ĵ����ܣ�kJ/mol���ֱ�Ϊ578��1817��2745��11575��EԪ��ԭ����4s�ܼ�����ȫ����״̬��
��1��A���⻯��е��ͬ��Ԫ�������⻯��е�ߵ�ԭ����
��2���о�������B��C���γ�һ����ԭ�ӷ��ӣ��Ҹ÷���������ԭ�Ӷ��ﵽ8�����ȶ��ṹ����÷��ӵĽṹʽΪ
��3��A��D��Ԫ������������Ӧ��ˮ�����ܷ�����Ӧ�������ӷ���ʽΪ
��4��E���⻯�ﳣ��Ұ���������ֻҪ������H2O�����������������H2��д���÷�Ӧ�Ļ�ѧ����ʽ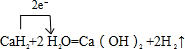
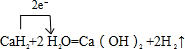 ��
��
��1��A���⻯��е��ͬ��Ԫ�������⻯��е�ߵ�ԭ����
NH3���Ӽ������
NH3���Ӽ������
�������ӹ�ҵ�У����⻯���ˮ��Һ������ʴ��H2O2�����������������Ӧ�IJ��ﲻ��Ⱦ�������仯ѧ����ʽΪ2NH3?H2O+3H2O2=N2��+8H2O
2NH3?H2O+3H2O2=N2��+8H2O
����2���о�������B��C���γ�һ����ԭ�ӷ��ӣ��Ҹ÷���������ԭ�Ӷ��ﵽ8�����ȶ��ṹ����÷��ӵĽṹʽΪ
F-O-F
F-O-F
����3��A��D��Ԫ������������Ӧ��ˮ�����ܷ�����Ӧ�������ӷ���ʽΪ
Al��OH��3+3H+=Al3++3H2O
Al��OH��3+3H+=Al3++3H2O
����4��E���⻯�ﳣ��Ұ���������ֻҪ������H2O�����������������H2��д���÷�Ӧ�Ļ�ѧ����ʽ
CaH2+2H2O=Ca��OH��2+2H2��
CaH2+2H2O=Ca��OH��2+2H2��
���õ����ű�ʾ����ת�Ƶķ������Ŀ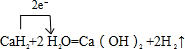
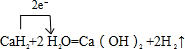
������A��B��C��D��E����Ԫ�أ����ǵ�ԭ���������������Ҷ������ڱ���ǰ20��Ԫ�أ�Aԭ�ӻ�̬ʱ��p�ܼ���������δ�ɶԵ��ӣ�ԭ�Ӻ�������Ų�Ϊ1s22s22p3��Ϊ��Ԫ�أ�BԪ�ص�ԭ�ӻ�̬ʱ��s�ܼ���p�ܼ��ĵ�������ȣ�ԭ�Ӻ�������Ų�Ϊ1s22s22p4����1s22s22p63s2��ΪOԪ�ػ�MgԪ�أ�B��Cͬ���ڣ���C�DZ������е縺������Ԫ�أ�Cλ�ڵڢ�A�壻Dԭ�ӵĵ�һ�����ĵ����ܣ�kJ/mol���ֱ�Ϊ578��1817��2745��11575�����������ܾ�������D����+3�ۣ�ԭ����������CԪ�أ���DΪAlԭ�ӡ�BΪ��Ԫ�ء�CΪFԪ�أ�EԪ��ԭ����4s�ܼ�����ȫ����״̬����EΪCaԪ�أ��ݴ˽��
����⣺A��B��C��D��E����Ԫ�أ����ǵ�ԭ���������������Ҷ������ڱ���ǰ20��Ԫ�أ�Aԭ�ӻ�̬ʱ��p�ܼ���������δ�ɶԵ��ӣ�ԭ�Ӻ�������Ų�Ϊ1s22s22p3��Ϊ��Ԫ�أ�BԪ�ص�ԭ�ӻ�̬ʱ��s�ܼ���p�ܼ��ĵ�������ȣ�ԭ�Ӻ�������Ų�Ϊ1s22s22p4����1s22s22p63s2��ΪOԪ�ػ�MgԪ�أ�B��Cͬ���ڣ���C�DZ������е縺������Ԫ�أ�Cλ�ڵڢ�A�壻Dԭ�ӵĵ�һ�����ĵ����ܣ�kJ/mol���ֱ�Ϊ578��1817��2745��11575�����������ܾ�������D����+3�ۣ�ԭ����������CԪ�أ���DΪAlԭ�ӡ�BΪ��Ԫ�ء�CΪFԪ�أ�EԪ��ԭ����4s�ܼ�����ȫ����״̬����EΪCaԪ�أ�
��1��AΪ��Ԫ�أ�A���⻯��Ϊ��������������֮�����������е��ͬ��Ԫ�������⻯��е�ߣ������ӹ�ҵ�У�������ˮ��Һ������ʴ��H2O2�����������������Ӧ�IJ��ﲻ��Ⱦ������Ӧ���ɵ�����ˮ����Ӧ����ʽΪ2NH3?H2O+3H2O2=N2��+8H2O��
�ʴ�Ϊ��NH3���Ӽ��������2NH3?H2O+3H2O2=N2��+8H2O��
��2���о�������O��FԪ�����γ�һ����ԭ�ӷ��ӣ��Ҹ÷���������ԭ�Ӷ��ﵽ8�����ȶ��ṹ��Ӧ��1����ԭ����2����ԭ�ӷֱ��γ�1�Թ��õ��Ӷԣ��ṹʽΪF-O-F��
�ʴ�Ϊ��F-O-F��
��3��A��D��Ԫ������������Ӧ��ˮ����ֱ�Ϊ���ᡢ�������������߷�Ӧ�����ӷ���ʽΪAl��OH��3+3H+=Al3++3H2O��
�ʴ�Ϊ��Al��OH��3+3H+=Al3++3H2O��
��4��E���⻯��ΪCaH2��������H2O�����������������H2��ͬʱ�����������ƣ�д���÷�Ӧ�Ļ�ѧ����ʽΪ��CaH2+2H2O=Ca��OH��2+2H2�����õ����ű�ʾ����ת�Ƶķ������ĿΪ��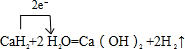 ��
��
�ʴ�Ϊ��CaH2+2H2O=Ca��OH��2+2H2����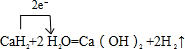 ��
��
��1��AΪ��Ԫ�أ�A���⻯��Ϊ��������������֮�����������е��ͬ��Ԫ�������⻯��е�ߣ������ӹ�ҵ�У�������ˮ��Һ������ʴ��H2O2�����������������Ӧ�IJ��ﲻ��Ⱦ������Ӧ���ɵ�����ˮ����Ӧ����ʽΪ2NH3?H2O+3H2O2=N2��+8H2O��
�ʴ�Ϊ��NH3���Ӽ��������2NH3?H2O+3H2O2=N2��+8H2O��
��2���о�������O��FԪ�����γ�һ����ԭ�ӷ��ӣ��Ҹ÷���������ԭ�Ӷ��ﵽ8�����ȶ��ṹ��Ӧ��1����ԭ����2����ԭ�ӷֱ��γ�1�Թ��õ��Ӷԣ��ṹʽΪF-O-F��
�ʴ�Ϊ��F-O-F��
��3��A��D��Ԫ������������Ӧ��ˮ����ֱ�Ϊ���ᡢ�������������߷�Ӧ�����ӷ���ʽΪAl��OH��3+3H+=Al3++3H2O��
�ʴ�Ϊ��Al��OH��3+3H+=Al3++3H2O��
��4��E���⻯��ΪCaH2��������H2O�����������������H2��ͬʱ�����������ƣ�д���÷�Ӧ�Ļ�ѧ����ʽΪ��CaH2+2H2O=Ca��OH��2+2H2�����õ����ű�ʾ����ת�Ƶķ������ĿΪ��
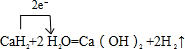 ��
���ʴ�Ϊ��CaH2+2H2O=Ca��OH��2+2H2����
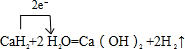 ��
�����������⿼��ṹ����λ�ù�ϵ�����û�ѧ����ȣ��Ѷ��еȣ��ƶ�Ԫ��Ϊ����ؼ����Ƕ�ѧ���ۺ������Ŀ��飮

��ϰ��ϵ�д�
�����Ŀ






 Ԫ�����ڱ������ڵ�һ���������ʾ�������й�A��B��C��D��E����Ԫ�ص������У���ȷ���ǣ�������
Ԫ�����ڱ������ڵ�һ���������ʾ�������й�A��B��C��D��E����Ԫ�ص������У���ȷ���ǣ�������