��Ŀ����
����Ŀ��������������Ԫ��֮һ������ֲ���纣���������к��зḻ�ġ��Ե�������ʽ���ڵĵ�Ԫ�ء���ʵ�����У��Ӻ�������ȡ������̺�ʵ��װ�����£�

����д���пհף�
(1)��������պ���ʱ������Ҫ���ż��⣬����Ҫ�õ���ʵ��������________(����ĸ)��
A �ձ� B ���� C ������ D ������ E �ƾ��� F ������
(2)����۵�ʵ�����������____________������ݵIJ���������________��
(3)����ܷ�Ӧ�����ӷ���ʽΪ_________________________________��
(4)�����һ�ּ�����ȡ����ˮ��Һ���Ƿ��е��ʵ�ļ�����______________________��
���𰸡�BDE ���� ��ȡ(����ȡ��Һ) Cl2 + 2I�� = 2Cl�� + I2 ȡ�������������Һ��������ɫ��֤���еⵥ��(�����CCl4��ȡ����Һ�����²����ɫ��֤���еⵥ��)
��������
��1�����������ͼ��֪���������յõ�����ң������������ˮ���ݵõ�����Һ������Һ���˵õ��������ӵ���Һ�������ӵ���Һ��ͨ���������������������ӷ����û���Ӧ���ɵⵥ�ʵõ����ⵥ�ʵ���Һ��Ȼ���ⵥ�ʵ���Һ�����л��ܼ���ȡ������ȡ��Һ�õ������ʵ���л���Һ������������õ����ʵ⡣
��1���������չ�������һ��ʹ�ã��ɣ����������������ż�����Ҫ������֧�ţ����ż�����žƾ��Ƽ��ȣ��ʴ�Ϊ��BDE��
��2��������Ƿ�������Һ�õ��������ӵ���Һ����������ʵ���������Ϊ���ˣ��������ⵥ�ʵ���Һ�����л��ܼ���ȡ������ȡ��Һ�õ������ʵ���л���Һ���ʴ�Ϊ�����ˣ���ȡ(����ȡ��Һ)��
(3)�������ķ�ӦΪ����������ӷ����û���Ӧ���ɵⵥ�ʣ���Ӧ�����ӷ���ʽΪCl2 + 2I�� = 2Cl�� + I2���ʴ�Ϊ��Cl2 + 2I�� = 2Cl�� + I2��
(4)����ȡ����ˮ��Һ�к��е��ʵ⣬���������Һ����Һ����ɫ�������CCl4���ã���Һ�ֲ㣬�²����ɫ���ʴ�Ϊ��ȡ�������������Һ��������ɫ��֤���еⵥ��(�����CCl4��ȡ����Һ�����²����ɫ��֤���еⵥ��)��

 ���Ӣ��������ϵ�д�
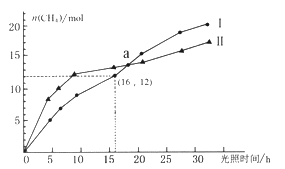
���Ӣ��������ϵ�д�����Ŀ����1����֪��CH4(g)+4NO2( g)=4NO(g)+CO2(g)+2H2O(g) ��H= -574 kJ��mol-l��CH4(g)+4NO(g)=2N2 (g)+CO2 (g)+2H2O(g) ��H=-1160 kJ��mol-1����������CH4��g����ԭNO2��g������N2��g����CO2��g����H2O(g)���Ȼ�ѧ����ʽ��__________��
(2)��֪��448 ��ʱ����ӦH2(g)��I2(g) ![]() 2HI(g)��ƽ�ⳣ��K1Ϊ49������¶��·�Ӧ��Ӧ
2HI(g)��ƽ�ⳣ��K1Ϊ49������¶��·�Ӧ��Ӧ![]() H2(g)��
H2(g)��![]() I2(g)
I2(g) ![]() HI(g)��ƽ�ⳣ��K3Ϊ___________________________��
HI(g)��ƽ�ⳣ��K3Ϊ___________________________��
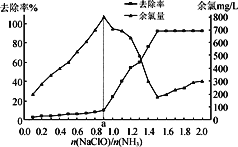
(3)��һ��������ܱ������н������»�ѧ��Ӧ��CO2(g)��H2(g) ![]() CO(g)��H2O(g)���仯ѧƽ�ⳣ��(K)���¶�(t)�Ĺ�ϵ���±���ʾ��
CO(g)��H2O(g)���仯ѧƽ�ⳣ��(K)���¶�(t)�Ĺ�ϵ���±���ʾ��
t/�� | 700 | 800 | 830 | 1 000 | 1 200 |
K | 0.6 | 0.9 | 1.0 | 1.7 | 2.6 |
�ش��������⣺
��)�÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪK��________��
�ڸ÷�ӦΪ________(��������������������)��Ӧ��
�����жϸ÷�Ӧ�ﵽ��ѧƽ��״̬��������________��
A.������ѹǿ���� B.���������c(CO)���� C.����(H2)������(H2O) D.c(CO2)��c(CO)
��ij�¶��£�ƽ��Ũ�ȷ�����ʽ��c(CO2)��c(H2)��c(CO)��c(H2O)�����жϴ�ʱ���¶�Ϊ________ ����
����800 ��ʱ������������Ӧ��ijһʱ�̲�������ڸ����ʵ�Ũ�ȷֱ�Ϊc(CO2)Ϊ2 mol��L��1��c(H2)Ϊ1.5 mol��L��1��c(CO)Ϊ1 mol��L��1��c(H2O)Ϊ3 mol��L��1������һʱ�̣���Ӧ��________(��������������������)���С�
����Ŀ��ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ�������ָʾ��������д���пհף�
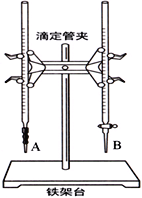
��1����ͼ��______���A����B�����Ǽ�ʽ�ζ��ܣ����и�ʵ��ĵ�һ��������____________________��
��2���ñ�������ζ������NaOH��Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע����ƿ����Һ��ɫ�仯��ֱ�������һ���������____________________________________�������˵���ﵽ�ζ��յ㡣
��3�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵�����____��
A����ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B���ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D����ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
��4�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ��������������Һ�����Ϊ________mL��
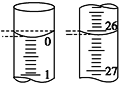
��5��ijѧ������3��ʵ��ֱ��¼�й��������±���
�ζ����� ����NaOH��Һ�����/mL | 0.100 0 mol��L��1��������/mL | ||
�ζ�ǰ�̶� | �ζ���̶� | ||
��һ�� | 25.00 | 0.00 | 26.11 |
�ڶ��� | 25.00 | 1.56 | 30.30 |
������ | 25.00 | 0.22 | 26.31 |
�����ϱ�����������NaOH��Һ�����ʵ���Ũ��__________________��