��Ŀ����
��1�����Ƕ�������ʶ�����м��������ʷ��������һ����dz����ɵͼ���������ʶ���̡�Ŀǰ��ѧ�α��е����������1889�갢������˹��Arrhenius������ĵ������ۡ�
��1905�긻�����֣�FranKlin�������о���ˮ��Һ���������ԣ��Ѱ�������˹��ˮΪ�ܼ��ĸ��������ƹ㵽�κ��ܼ������������ܼ����ۡ��ܼ�������Ϊ�����ܵ���������ܼ������ӵ�����Ϊ�ᣬ���ܵ���������ܼ������ӵ�����Ϊ���д��Һ����������ķ���ʽ�� ��
��1923�굤��ѧ�Ҳ���˹�أ�Bronsted����Ӣ����ѧ��������Lowey������������ۡ������ܹ��ͷ����ӣ������ӣ��κκ���ԭ�ӵķ��ӻ����Ӷ���������ܹ������ӣ������ӣ���ϵķ��ӻ����Ӷ��Ǽ
���������ۣ�����������ˮ��Һ�ȿ��Կ������ֿɿ������ ��
A��H2O B��NH4+ C��OH- D��HCO3- E��CH3COO- F��Cl-
��1923��·��˹��Lewis��������������������ܸ������ӶԶ��γɻ�ѧ�������ʶ��Ǽ�����ܹ��͵��ӶԽ�ϵ����ʶ����ᡣ
�ᣨ���ӶԽ����壩+����ӶԸ����壩����Ӧ����磺H++OH����H2O��
��ָ������������Ӧ�е����
(��)H3BO3 +H2O H++B(OH)4-���÷�Ӧ�еļ��� ����H3BO3��H2O����
H++B(OH)4-���÷�Ӧ�еļ��� ����H3BO3��H2O����
(��)NaH+H2O ==NaOH+H2�����÷�Ӧ�е����� ����NaH ��H2O����
��2����֪AԪ��ԭ�ӵ�K��L�������֮�ͱ�M��L�������֮�Ͷ�һ�����ӣ�BԪ��ԭ�Ӻ������ռ��9�����������1��δ�ɶԵ��ӣ�CԪ��ԭ�Ӻ���3p�Dz���3���������5�����Ӳ��ܴﵽȫ������DԪ��ֻ���������Ӳ㣬������ϼ�����ͻ��ϼ۵Ĵ�����Ϊ�㣻Eԭ�Ӱ뾶��С��FԪ�����������Ų�ΪnSnnPn+1����Ҫ����д
�� B�ĵ����Ų�ʽ�ǣ� ��A��B��Ԫ���γɵĻ�����ľ��������� ��
��DԪ�ص��ʵľ��������� ��C���ʵ��Ʊ������ǣ� ��
��E��F�γɵĻ�����ռ乹��Ϊ �������ʱ�D��E�γɵĻ����������Һ����ԭ���� ��
��1905�긻�����֣�FranKlin�������о���ˮ��Һ���������ԣ��Ѱ�������˹��ˮΪ�ܼ��ĸ��������ƹ㵽�κ��ܼ������������ܼ����ۡ��ܼ�������Ϊ�����ܵ���������ܼ������ӵ�����Ϊ�ᣬ���ܵ���������ܼ������ӵ�����Ϊ���д��Һ����������ķ���ʽ�� ��
��1923�굤��ѧ�Ҳ���˹�أ�Bronsted����Ӣ����ѧ��������Lowey������������ۡ������ܹ��ͷ����ӣ������ӣ��κκ���ԭ�ӵķ��ӻ����Ӷ���������ܹ������ӣ������ӣ���ϵķ��ӻ����Ӷ��Ǽ
���������ۣ�����������ˮ��Һ�ȿ��Կ������ֿɿ������ ��
A��H2O B��NH4+ C��OH- D��HCO3- E��CH3COO- F��Cl-
��1923��·��˹��Lewis��������������������ܸ������ӶԶ��γɻ�ѧ�������ʶ��Ǽ�����ܹ��͵��ӶԽ�ϵ����ʶ����ᡣ
�ᣨ���ӶԽ����壩+����ӶԸ����壩����Ӧ����磺H++OH����H2O��
��ָ������������Ӧ�е����
(��)H3BO3 +H2O
 H++B(OH)4-���÷�Ӧ�еļ��� ����H3BO3��H2O����
H++B(OH)4-���÷�Ӧ�еļ��� ����H3BO3��H2O����(��)NaH+H2O ==NaOH+H2�����÷�Ӧ�е����� ����NaH ��H2O����
��2����֪AԪ��ԭ�ӵ�K��L�������֮�ͱ�M��L�������֮�Ͷ�һ�����ӣ�BԪ��ԭ�Ӻ������ռ��9�����������1��δ�ɶԵ��ӣ�CԪ��ԭ�Ӻ���3p�Dz���3���������5�����Ӳ��ܴﵽȫ������DԪ��ֻ���������Ӳ㣬������ϼ�����ͻ��ϼ۵Ĵ�����Ϊ�㣻Eԭ�Ӱ뾶��С��FԪ�����������Ų�ΪnSnnPn+1����Ҫ����д
�� B�ĵ����Ų�ʽ�ǣ� ��A��B��Ԫ���γɵĻ�����ľ��������� ��
��DԪ�ص��ʵľ��������� ��C���ʵ��Ʊ������ǣ� ��
��E��F�γɵĻ�����ռ乹��Ϊ �������ʱ�D��E�γɵĻ����������Һ����ԭ���� ��
��1����4�֣���2NH3 = NH4+ + NH2- �� A D �ۣ���H2O ����H2O
��2����6�֣��� 1s22s22p63s23p5 ���Ӿ��� ��ԭ�Ӿ��� ��ⷨ
�������� E��F�γɵĻ�������Ӽ����γ����
��2����6�֣��� 1s22s22p63s23p5 ���Ӿ��� ��ԭ�Ӿ��� ��ⷨ
�������� E��F�γɵĻ�������Ӽ����γ����
��

��ϰ��ϵ�д�
 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�
�����Ŀ
 H3O+ + CO32��
H3O+ + CO32��
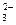 )>C(HCO
)>C(HCO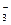 )>C(OH-)>C(H+)
)>C(OH-)>C(H+)