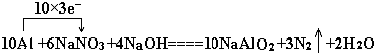��Ŀ����
20��ij����ѧϰС���ͬѧ������ɰ��Na2B4C7•l0H2O����ȡ���ᣨH3B03�����ⶨ������Ʒ�Ĵ��ȣ��Ʊ������ʵ���������£�
��1����pH��ֽ�ⶨ��ҺpH�IJ�������Ϊ��������
��2������aʵ��������貣��������©�������������ձ���
��3��ʵ��������ȱ�ٵIJ���b����Ϊϴ�ӣ�
��4����������̫�������ü�ı���Һֱ�ӵζ���ʵ���ҳ����ü�ӵζ�������ԭ��Ϊ��
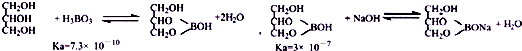
�������
ȷ��ȡ0.3000g��Ʒ����ƿ$��_{25mL}^{���Ը���}$$��_{�ܽ�}^{����}$��ȴ$\stackrel{����1-2�η�̪}{��}$$��_{�ζ�}^{0.2000mol•L-1NaOH��Һ}$�ζ��յ�
�ٵζ��յ���жϷ���������ɫ��Ϊdz��ɫ��
�����ζ����յ�ʱ����NaOH����Һ22.00mL���εζ���õ�������Ʒ���������������Ϊ90.93%���ٶ����ʲ���Ӧ����
�����ζ�ǰ�ζ��ܼ��첿�������ݣ��ζ���������ʧ�������²�õĽ��ƫ��ѡ���ƫ ����ƫС�����䡱����
���� ����ɰ��ȡ���Ტ�ⶨ������Ʒ�Ĵ��ȣ���ɰ�ܽ��������������ҺPHΪ3-4����ȴ��9��C�����½ᾧ������ϴ�Ӹ���õ����ᣬ
��1�������ܽ���Ҫ�ձ��Ͳ�������
��2������ʵ�����������Ҫ�IJ���������
��3����������������֪��������b��ϴ�ӣ�
��4���ٵζ������м����ָʾ���Ƿ�̪��Һ����������������Һ����Ӧ�յ㣬��Һ��ɫ����ɫ�仯Ϊdz��ɫ֤����Ӧ�ﵽ�յ㣻
�ڷ����ζ�ԭ���ͷ�Ӧ�Ķ�����ϵ���㣻
�����ζ�ʱ�ζ��ܼ��첿�������ݣ��ζ���������ʧ�������ı���Һ�����������c�����⣩=$\frac{c������V������}{V�����⣩}$���ⶨ���ƫ��
��� �⣺����ɰ��ȡ���Ტ�ⶨ������Ʒ�Ĵ��ȣ���ɰ�ܽ��������������ҺPHΪ3-4����ȴ��9��C�����½ᾧ������ϴ�Ӹ���õ����
��1�������ܽ���Ҫ�ձ��Ͳ��������ܽ���ɰʱ��Ҫ�IJ��������У��ձ��Ͳ�������
�ʴ�Ϊ����������
��2������aʵ�����Ϊ���˲��������貣��������©�������������ձ���
�ʴ�Ϊ��©�������������ձ���
��3����������������֪ʵ��������ȱ�ٵIJ���a������b�����ֱ�Ϊ���ˡ�ϴ�ӣ�
�ʴ�Ϊ��ϴ�ӣ�
��4���ٷ������̿�֪���ζ������м����ָʾ���Ƿ�̪��Һ����������������Һ����Ӧ�յ������Ϊ���������һ����Һ��ɫ����ɫ�仯Ϊdz��ɫ�Ұ���Ӳ��仯��֤����Ӧ�ﵽ�յ㣬�ζ����յ�ʱ��Һ��ɫ�仯������ɫ��Ϊdz��ɫ��
�ʴ�Ϊ������ɫ��Ϊdz��ɫ��
�ڷ����ζ�ԭ���ͷ�Ӧ�Ķ�����ϵ���㣬ʵ���ҳ����ü�ӵζ�������ԭ��Ϊ�� ��
��
���ζ����յ�ʱ����NaOH����Һ22.00mL������������ҺŨ��Ϊ0.2000mol/L��
H3BO3��NaOH
1 1
n 0.0220L��0.2000mol/L
n=0.0044mol
�εζ���õ�������Ʒ���������������=$\frac{0.0044mol��62g/mol}{0.3000g}$��100%=90.93%��
�ʴ�Ϊ��90.93%��
�����ζ�ʱ�ζ��ܼ��첿�������ݣ��ζ���������ʧ�������ı���Һ������ⶨ���ƫ��
�ʴ�Ϊ��ƫ��
���� ���⿼���������Ʊ������̷����жϣ��������ʺ�ʵ������жϣ��ζ�ʵ��IJ������̺ͼ���Ӧ�ã����ջ����ǹؼ�����Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | �۲�Ƚ������̬ | B�� | ���ִ����Ƚ� | ||
| C�� | �ŵ�����Ʒ��һ�� | D�� | �û�ѧ�������м��� |
 |  |  |  | |
| ���� | �ȼҵ | ��¯���� | ͭ�ľ��� | ����Ư�� |
| ԭ�� | 2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$ 2NaOH+Cl2��+ H2�� | 3CO+Fe2O3 $\frac{\underline{\;����\;}}{\;}$2Fe+3CO2 | ������ Cu2++2e-�TCu | 2NaOH+Cl2�TNaCl+NaClO+H2O |
| A | B | C | D |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ������Ӧʽ��Ag+Cl-+e-�TAgCl | |
| B�� | Na+������ˮ����ص������ƶ� | |
| C�� | ÿ����1molNa2Mn5O10ת��2mol���� | |
| D�� | AgCl�ǻ�ԭ���� |
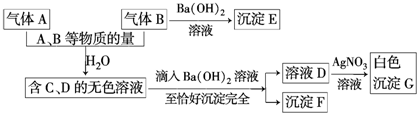
��ijС���ú��������������������������ʵĴ���ʯ��ȡ�������Ƶ��������£�
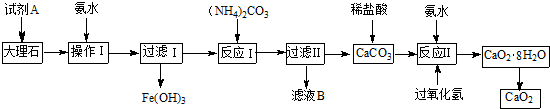
��ش��������⣺
��1������I��Ŀ���ǵ�����Һ��pH��ʹ��Ԫ����ȫ���������Լ�A���ѡ��c��
a������ b������ c������������� d������
��2���������I����Ԫ���ѳ�����ȫ�IJ�����ȡ�ϲ���Һ�������Թ��У��μ�KSCN��Һ������Һ����죬����Ԫ���ѳ�����
��3����ҺB��Ҫ�ɷֵĻ�ѧʽ��NH4Cl��
��4����Ӧ���Ƿ��ȷ�Ӧ������H2O2���ʵ���һ������H2O2Ũ��С��20%ʱ��CaO2�IJ�����H2O2Ũ�ȵ����������Ũ�ȴ���20%��CaO2���ʷ����½����Է���CaO2�����½��Ŀ���ԭ����H2O2Ũ�ȸߣ���Ӧ���ʿ죬��Ӧ����ʹ��ϵ����Ѹ�٣���ʹH2O2�ֽ⣮
���������г�����CaO���ʣ�ʵ���ҿɰ����²���ⶨCaO2������
����1��ȷ��ȡ0.3900g����������Ʒ������250mL����ƿ�У�
����2���ֱ����10mL����ˮ��20mL���ᣨ1��3������ʹ��Ʒ��ȫ�ܽ⣻
����3����0.1000mol•L-1 KMnO4����Һ�ζ����յ㣬��¼���ݣ�
����4��ƽ�вⶨ4�Σ����ݼ�¼���±���������������CaO2������������
| ʵ�� | 1 | 2 | 3 | 4 |
| V��KMnO4��/mL | 19.50 | 21.50 | 19.48 | 19.52 |
��ش��������⣺
��5������3�жϵζ��ﵽ�յ�������ǵ���������1��KMnO4��Һ����ƿ����Һ����ɫ��Ϊdz��ɫ����ۺ�ɫ����ɫ����30s����ɫ�����ﵽ�ζ��յ㣻
��6���ɱ������ݿ�֪������Ʒ��CaO2����Ϊ90%����ʵ��ʱ��ϴ���ĵζ���δ��KMnO4����Һ��ϴ����CaO2�����������ⶨ���ƫ�ߣ��ƫ�ߡ�����ƫ�͡�����Ӱ�족����
| A�� | ���Ǻ�̼Ԫ�صĻ����ﶼ�����л��� | |
| B�� | ���������͡��ƾ��������л��ܼ��е�����һ�����л��� | |
| C�� | ���е��л��ﶼ������ȼ�� | |
| D�� | �л����������ķ�Ӧ��һ��Ƚϸ��ӣ��ٶȻ��������һ������и���Ӧ���� |
| A�� | 1.6a g | B�� | ��a-1.6��g | C�� | ��a-3.2��g | D�� | ������ |