��Ŀ����
��14�֣� ij�о�С�������������о���
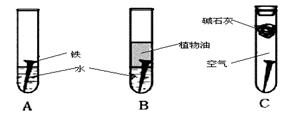
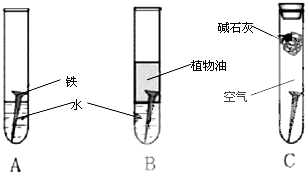
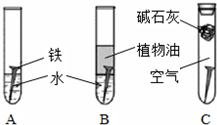
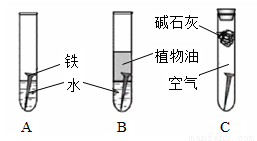
��1�������ϳ�ʱ���ͬѧ�۲쵽�������ǣ���ͼ�е������������������ ������ĸ�����������Ҫ�ɷ���
��2������ʵ�������жϣ�����������ʴ�����У������ĵ缫��ӦΪ
��3����������ⲿ������ ���ڴ������£���������������ʴת��ΪFe��OH��2�ĵ�ط�Ӧ����ʽΪ
��4����Ϊ�˷�ֹ�������⣬��С��ͬѧ���������������һ��������ý��������
A. �� B. ͭ C. п
��5����������ʴ����ҵ�����г���Ը������С�����������������Ч�������������ĸ�ʴ����ν���������������ڸ�������Ƚ�������������ʹ������γ�һ�����ܵ�����ɫ����Ĥ�������������̿ɱ�ʾ���£�
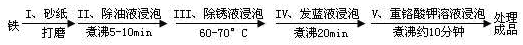
�� ������ó���Һ��15%��������Һ������������Ŀ�����ڳ�ȥ����������⣬�ò���Ӧ�����ӷ���ʽΪ___________________________________��
�� Ϊ���龭������������������Ƿ�ϸ�����Ʒ�������5%������ͭ��Һ�������Ʒ���ϸ�����������С�ɿף�δ�γ����ܵ�����Ĥ����һ��ʱ�佫�۲쵽������Ϊ__________________________��
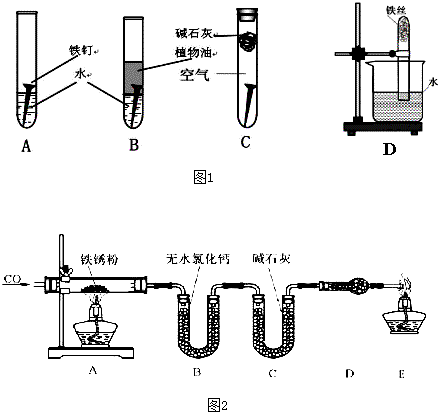
�� �����������ڷ���Һ��NaNO2��NaNO3��NaOH��ɵĻ��Һ���н��ݣ�����IV���������˸��ӵĻ�ѧ��Ӧ��
��Ӧһ��____Fe ��____NaNO2 ��___NaOH ��____Na2FeO2 ��____H2O ��___NH3��
��Ӧ����8Fe��3NaNO3 �� 5NaOH �� 2H2O �� 4Na2Fe2O4 �� 3 NH3��
��Ӧ����Na2FeO2 �� Na2Fe2O4 �� 2H2O �� Fe3O4 �� 4NaOH
��ƽ����Ӧһ���Ļ�ѧ����ʽ����ϵ��ֱ�����ں����ϣ�����Ҫѭ��ʹ�÷���Һ�������۽Ƕȷ�������Ҫ��ʹ�ù��ķ���Һ��_________
A. ֻ�����NaNO2 B. ֻ�����NaNO2��NaNO3
C. ��Ҫ����NaNO2��NaNO3��NaOH D. ��������κ����ʶ�ֱ��ʹ��
��14�֣���1��A�� Fe2O3���� Fe2O3�� xH2O��
��2��O2��2 H2O��4e���� 4 OH��
��3����ʪ�Ŀ��� ����������ˮ�� �� 2Fe ��O2 ��2 H2O��2 Fe(OH)2
��4��C ��5���� Fe2O3��6 H�� ��2 Fe3����3 H2O
�� ����Ʒ�����к�ɫ���������� 3��1��5��3��1��1��B
����������1��B��C�����ܸ�����������ֹ�������绯ѧ��ʴ����A���ܣ����������ױ���ʴ����A���������Ҫ�ɷ���Fe2O3���� Fe2O3�� xH2O����
��2�������ĵ绯ѧ��ʴ�У�����������������Һ�����Ժ��������������������õ����ӣ�����ӦʽΪO2��2 H2O��4e���� 4 OH����
��3����Ҫ��ʴ�绯ѧ��ʴ������봦�ڳ�ʪ�Ŀ��� ����������ˮ������Ӧ���ܷ�Ӧʽ��2Fe ��O2 ��2 H2O��2 Fe(OH)2.
(4)���ڶƲ�һ������֮������ʴ�绯ѧ��ʴ���������ƽ����Ա���ǿ�Ľ��������Դ�ѡC��
��5�����������Ҫ�ɷ�������������ϡ���ᷴӦ�ķ���ʽΪFe2O3��6 H�� ��2 Fe3����3 H2O��
����Ϊ���Ļ�����ǿ��ͭ�����ܺ�����ͭ��Ӧ�û���ͭ����������Ʒ�����к�ɫ����������
���ڷ�Ӧ����ʧȥ��������ԭ�������ϼ۴�0�����ߵ���2�ۡ����������������ƣ���Ԫ�صĻ��ϼ۴ӣ�3�۽��͵���3�ۣ��仯6����λ�����������ͻ�ԭ�������ʵ���֮����1�U3�ģ����Է���ʽΪ3Fe ��NaNO2 ��5NaOH��3Na2FeO2 ��H2O ��NH3������3����Ӧ�ϲ����õ�12Fe��NaNO3��4NaNO2��10H2O=4Fe3O4��5NH3����5NaOH����˵����Ӧ�����ĵ��������ƺ��������ƣ�������Ҫ������ߣ���ѡB��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�