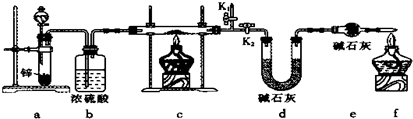
��Ŀ����
(1)�⻯��ͭ(CuH)��һ���������ʣ���CuSO4��Һ�͡���һ���ʡ���40������������Ϣ������Լ������յĻ�ѧ֪ʶ���ش��������⣺
����CuSO4��Һ�͡���һ���ʡ���CuH�ķ�Ӧ�У���������ԭ�۵�������⡰��һ���ʡ��ڷ�Ӧ�������������_______________(�����������ԭ����)��
�������CuH�ܽ���������ϡ���������ɵ�����ֻ��NO����д��CuH�ܽ�������ϡ�����з�Ӧ�Ļ�ѧ����ʽ(������ƽ)��_________________________________���÷�Ӧ�е�����������________________��
(2)��ͼ�У�PΪһ���������ɻ����Ļ������ر�K���ֱ���A��B�������и�����2 mol X��2 mol Y������ͬ�¶Ⱥ��д������ڵ������£��������и��Է������·�Ӧ��

2X(g)+Y(g)![]() 2Z(g)+2W(g)
2Z(g)+2W(g)
��֪����ʼʱ��VA=
��A��B�������ﵽƽ���ʱ��tA________________tB(����ڡ���С�ڡ����ڡ�)��
�ڼ�����¶��·�Ӧ�Ļ�ѧƽ�ⳣ��K=___________��
�۵�A��B�ֱ�ﵽƽ��ʱ��B������Y��ת����Ϊ____________����������W�����������ϵΪA____________B(����ڡ���С�ڡ����ڡ�)��
�ܴ�K��һ��ʱ���Ӧ�ٴδﵽƽ�⣬���ʱB�����Ϊ____________��
(1)�ٻ�ԭ��
��CuH+3HNO3![]() Cu(NO3)2+NO��+2H2O Cu(NO3)2��H2O
Cu(NO3)2+NO��+2H2O Cu(NO3)2��H2O
(2)�ٴ��� ��0.74 mol��L-1 ��25% ���� ��0.8 L
������(1)![]() ��Cu�Ļ��ϼ۽��ͣ���һ�����ʵĻ��ϼۿ϶����ߣ�������һ�������ڷ�Ӧ������ԭ����
��Cu�Ļ��ϼ۽��ͣ���һ�����ʵĻ��ϼۿ϶����ߣ�������һ�������ڷ�Ӧ������ԭ����
![]()
����������Cu(NO3)2��H2O��
(2)Ũ��Խ��Ӧ����Խ�죬����V(A)=1 L��V(B)=0.9 L������tA��tB��
2X(g)+Y(g)![]() 2Z(g)+2W(g)
2Z(g)+2W(g)
��ʼ���ʵ���/mol 2 2 0 0
ת�����ʵ���/mol 2x x 2x 2x
ƽ�����ʵ���/mol 2-2x 2-x 2x 2x
![]() x=0.5
x=0.5
K=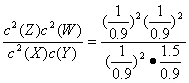 mol��L-1=0.74 mol��L-1
mol��L-1=0.74 mol��L-1
Y��ת����Ϊ![]() ��100%=25%
��100%=25%
A�����൱����B�����Ļ����ϼ�Сѹǿ�����������ƽ�������ƶ���W���������A��B��
����K���൱��A��B����Ϊ��ѹ�µ��������ﵽƽ��ʱ��V(A)=V(B)=0.9 L��V (A)+V(B)=