��Ŀ����
����������Ϣ�ش��������⡣
�� .�μ�أ����϶�NaCl��Na2CO3���������������ɣ�ͨ��ʩ������ʯ�ࣨCaSO4�����Խ��������ļ��ԡ�
.�μ�أ����϶�NaCl��Na2CO3���������������ɣ�ͨ��ʩ������ʯ�ࣨCaSO4�����Խ��������ļ��ԡ�
��.����ʹ�õĹ�¯��Ҫ���ڳ�ˮ��������ή��ȼ�ϵ������ʡ�ˮ���к��е�CaSO4��������Na2CO3��Һ������ʹ֮ת��Ϊ���ɡ����������CaCO3�������������ȥ��
���������ӷ���ʽ��ʾ�μ�ز������Ե�ԭ�� ��
�����û�ѧ����ʽ��ʾ ����ʯ�ཱུ���������Եķ�Ӧԭ�� ��
����ʯ�ཱུ���������Եķ�Ӧԭ�� ��
����д��ˮ������Һ��CaSO4�ܽ�ƽ������ӷ���ʽ ��
�� �����ˮ���е�CaSO4ת��ΪCaCO3��ԭ�� ��
�����ˮ���е�CaSO4ת��ΪCaCO3��ԭ�� ��
����д��CaCO3���ڹ�����������ӷ���ʽ ��
��
 .�μ�أ����϶�NaCl��Na2CO3���������������ɣ�ͨ��ʩ������ʯ�ࣨCaSO4�����Խ��������ļ��ԡ�
.�μ�أ����϶�NaCl��Na2CO3���������������ɣ�ͨ��ʩ������ʯ�ࣨCaSO4�����Խ��������ļ��ԡ���.����ʹ�õĹ�¯��Ҫ���ڳ�ˮ��������ή��ȼ�ϵ������ʡ�ˮ���к��е�CaSO4��������Na2CO3��Һ������ʹ֮ת��Ϊ���ɡ����������CaCO3�������������ȥ��
���������ӷ���ʽ��ʾ�μ�ز������Ե�ԭ�� ��
�����û�ѧ����ʽ��ʾ
 ����ʯ�ཱུ���������Եķ�Ӧԭ�� ��
����ʯ�ཱུ���������Եķ�Ӧԭ�� ������д��ˮ������Һ��CaSO4�ܽ�ƽ������ӷ���ʽ ��
��
 �����ˮ���е�CaSO4ת��ΪCaCO3��ԭ�� ��
�����ˮ���е�CaSO4ת��ΪCaCO3��ԭ�� ������д��CaCO3���ڹ�����������ӷ���ʽ ��
��CO32-+H2O 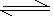 HCO3-+OH-, HCO3-+H2O
HCO3-+OH-, HCO3-+H2O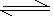 H2CO3+OH-
H2CO3+OH-
��CaSO4+Na2CO3=CaCO3+ Na2SO4
�� CaSO4(s)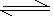 Ca2+(aq)+SO42-(aq)
Ca2+(aq)+SO42-(aq)
��CaSO4�ij����ܽ�ƽ�⣬����Na2CO3��Һ��CO32- ��Ca2+������ɸ�������ˮ��CaCO3������Ca2+Ũ�ȼ��٣�ʹCaSO4�ij����ܽ�ƽ�����ܽⷽ���ƶ���CaSO4�������٣�CaCO3�������ɡ�
��CaCO3 +2H+= CO2��+ H2O +Ca2+
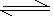 HCO3-+OH-, HCO3-+H2O
HCO3-+OH-, HCO3-+H2O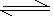 H2CO3+OH-
H2CO3+OH-��CaSO4+Na2CO3=CaCO3+ Na2SO4
�� CaSO4(s)
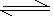 Ca2+(aq)+SO42-(aq)
Ca2+(aq)+SO42-(aq)��CaSO4�ij����ܽ�ƽ�⣬����Na2CO3��Һ��CO32- ��Ca2+������ɸ�������ˮ��CaCO3������Ca2+Ũ�ȼ��٣�ʹCaSO4�ij����ܽ�ƽ�����ܽⷽ���ƶ���CaSO4�������٣�CaCO3�������ɡ�
��CaCO3 +2H+= CO2��+ H2O +Ca2+
��

��ϰ��ϵ�д�
 ��ѧ��������������Ͼ���ѧ������ϵ�д�
��ѧ��������������Ͼ���ѧ������ϵ�д� �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
�����Ŀ


 ��������������ȷ���� �� ��
��������������ȷ���� �� ��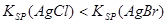
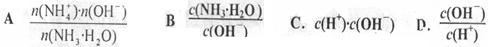
 4)��________��
4)��________��