��Ŀ����
����Ŀ��4Na2SO42H2O2NaCl�ֳƹ���˫��ˮ������Ư�ס�ɱ�����������ã����������ȶ��Աȹ�̼����(2Na2CO33H2O2)������ã�������й㷺��Ӧ��ǰ����ijС��ϳɸù���˫��ˮ��ʵ�鲽���װ��ʾ��ͼ���£�
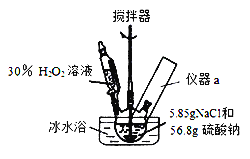
��.�ϳɣ�������ƿ�м���56.8g�����ƺ�5.85gNaCl�Ĺ���������������������Թ�����(Լ70mL)30%H2O2��Һ��20��30min����η������롣�����Ʒ�Ӧ�¶�15��35�棬�����Ϻ��������15min����Ӧ��������ˣ����¸���õ���Ʒ��
��.��Ʒ�ȶ���ȡ�������ò�Ʒ���ڸ������ڱ���һ���£����ֱ��ڷ���ǰ�����ú�ȡһ����������Ʒ����ˮ��������ϡ�����ữ����0.1000mol/L���Ը�����صζ����ⶨ��Ʒ��˫��ˮ�ĺ������Դ˷�����Ʒ���ȶ��ԣ�����ʵ���������±���
���� ʱ�� | ��Ʒȡ������(g) | ƽ��V(KMnO4)/mL |
����ǰ�ⶨ | a | 25.00 |
����һ���º�ⶨ | a | 24.90 |
��֪��a.H2O2���ȶ������ȣ�����ijЩ�������ӻ�Ӽ����������ֽ�
b.��Ʒ�ȶ���=![]() ��100%
��100%
��ش��������⣺
(1)װ��ͼ�﷽����Ӧ��������a��������Ϊ_______________________��д���ϳ�4Na2SO42H2O2NaCl�Ļ�ѧ����ʽ��_____________________________��
(2)�úϳɷ�Ӧ�����У��ؼ��ǿ����¶ȣ�������Ĵ�ʩ�ǣ�______________��
A.��εμ�H2O2 B.ѡ��Na2SO4��NaCl���壬�������䱥����Һ
C.���Ͻ��� D.��ˮԡ
(3)�úϳɷ�Ӧ������30%��H2O2��Һ��Ӧ��������ԭ��________________��
(4)4Na2SO42H2O2NaCl����ȹ�̼����(2Na2CO33H2O2)������ȶ��Ŀ���ԭ����__________��
(5)��Ʒ����ʵ��ʱ�����������Һװ�ڵζ����У����ζ������յ�ʱ��������_________________�����й��ڸõζ�ʵ������е�����ѡ��Ͳ�������ȷ����_____________
A.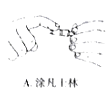 B.
B. C.
C. D.
D.
(6)�ò�Ʒ������Ʒ�ȶ�����=___________________��
���𰸡��¶ȼ� 4Na2SO4+2H2O2+NaCl=4Na2SO42H2O2NaCl ACD ��С��Ʒ���ܽ���ʧ����߲��� 2Na2CO33H2O2��̼����ˮ��ʼ��ԣ���˫��ˮ�ڼ����������ֽ� ��Һ����ɫ�պñ�Ϊdz��ɫ���ұ���30s����ɫ AC 99.6%
��������
(1)���ݿ��Ʒ�Ӧ�¶�15��35���֪��װ���б���Ҫ���¶ȼƣ����������ṩ�����ʣ�����Ԫ���غ��д��ѧ����ʽ��
(2)���Ʒ�Ӧ�ų�����������ͨ�����Ʒ�Ӧ�������Ҳ����ͨ�����衢��ˮԡ�ȷ�ʽ��ʹ����ɢʧ�ȴ�ʩ��
(3)��Ϊ˫��ˮ���ȶ������Ժϳɷ�Ӧ������30%��H2O2��Һ�ʵ�������������̫�࣬˫��ˮ̫�࣬4Na2SO42H2O2NaCl�����е��ܽ�����Ͷ࣬��Ʒ�IJ��ʵͣ�
(4)2Na2CO33H2O2��̼����ˮ��ʼ��ԣ���˫��ˮ�ڼ����������ֽ⣻
(5)���ݸ��������Һ��������ɫ�ı仯�жϵζ����յ㣬���ݵζ������Ĺ淶Ҫ��ѡ��
(6)���ݲ�Ʒ��ȥ�ĸ��������Һ������ɼ������Ʒ��˫��ˮ���������������ò�Ʒ�ȶ���=![]() ��100%��
��100%��
(1)���ݿ��Ʒ�Ӧ�¶�15��35���֪��װ���б���Ҫ���¶ȼƣ����Է�����Ӧ��������aΪ�¶ȼƣ��ϳ�4Na2SO42H2O2NaCl�Ļ�ѧ����ʽΪ4Na2SO4+2H2O2+NaCl=4Na2SO42H2O2NaCl��
(2)���Ʒ�Ӧ�ų�����������ͨ�����Ʒ�Ӧ�������Ҳ����ͨ�����衢��ˮԡ�ȷ�ʽ��ʹ����ɢʧ�ȴ�ʩ�ﵽ�����¶ȵ�Ŀ�ģ��ʺ���ѡ����ACD��
(3)��Ϊ˫��ˮ���ȶ������Ժϳɷ�Ӧ������30%��H2O2��Һ�ʵ�������������̫�࣬˫��ˮ̫�࣬4Na2SO42H2O2NaCl�����е��ܽ�����Ͷ࣬��Ʒ�IJ��ʵͣ�����30%��H2O2��Һ��Ӧ���������Լ�С��Ʒ���ܽ���ʧ����߲��ʣ�
(4)2Na2CO33H2O2��̼����ˮ��ʼ��ԣ���˫��ˮ�ڼ����������ֽ⣬����4Na2SO42H2O2NaCl����ȹ�̼����(2Na2CO33H2O2)������ȶ���
(5)A.���������Һװ����ʽ�ζ����У����ζ������յ�ʱ�����������ǣ���Һ����ɫ�պñ�Ϊdz��ɫ���ұ���30s����ɫ������ʽ�ζ��ܵĻ�����Ϳ��ʿ�ֿ��Է�ֹ����©Һ�壬A��ȷ��
B.���������ҺӦ����ʽ�ζ���ʢ�ţ���ʽ�ζ����ų�����Ӧ�ÿ��ٷ�Һ����B����
C.�ζ�ʱ���ֿ�����ʽ�ζ��ܵĻ�����������ס��ƿ����������C��ȷ��
D.������Ŀ��Ҫƽ��Һ�棬���ܸ��ӣ�D����
�ʺ���ѡ����AC��
(6)���ݲ�Ʒ��ȥ�ĸ��������Һ������ɼ������Ʒ��˫��ˮ�����������������õ���ͬһ����Ʒ�������и��������Һ�����֮��=˫��ˮ����������֮�ȣ����Բ�Ʒ�ȶ���![]() ��100%=
��100%=![]() ��100%=99.6%��
��100%=99.6%��

 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�����Ŀ�������й����ʵ��Ʊ�װ�á��Ʊ�ʱʹ�õ�ԭ�����ռ���������ȷ���ǣ� ��
��� | ���� | �Ʊ�װ�� | �Ʊ�ʱʹ�õ�ԭ�� | �ռ����� | ��� | ���� | �Ʊ�װ�� | �Ʊ�ʱʹ�õ�ԭ�� | �ռ����� |
A |
|
|
| ��ˮ�� | B | NO |
| Ũ����ͽ���ͭ | �ſ����� |
C |
|
| Ũ��ˮ���������ƹ��� | �ſ����� | D |
|
| �Ҵ��� | ��ˮ�� |
A.AB.BC.CD.D