��Ŀ����
ijѧϰС��������ͼ��ʾװ�òⶨ��þ�Ͻ������������������������ԭ��������

��1��A���Լ�Ϊ__________��
��2��ʵ��ǰ���Ƚ���þ�Ͻ���ϡ���н���Ƭ�̣���Ŀ����____________________��
��3����������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�ú�����еIJ������У��ټ�¼C��Һ��λ�ã��ڽ�B��ʣ�������ˣ�ϴ�ӣ�������أ��۴�B�в���������������ָ������º�¼C��Һ��λ�ã�����A��B�μ������Լ����ݼ�������ԡ�����������˳����__________������ţ�����¼C��Һ��λ��ʱ��������ƽ���⣬��Ӧ__________��
��4��B�з�����Ӧ�Ļ�ѧ����ʽΪ____________________��
��5����ʵ������þ�Ͻ������Ϊa g������������Ϊb mL���ѻ���Ϊ��״������B��ʣ����������Ϊc g?�����������ԭ������Ϊ__________��
��6��ʵ������У���δϴ�ӹ������õIJ�������õ�����������__________���ƫ��ƫС������Ӱ�족����
��1��NaOH��Һ
��2����ȥ��þ�Ͻ���������Ĥ
��3���ݢ٢ܢۢڡ�ʹD��C��Һ����ƽ
��4��2Al��2NaOH��2H2O ==== 2NaAlO2��3H2��
��5��![]() ����6��ƫС
����6��ƫС
����:
��1���۲�ʵ��װ��ͼ��֪�������������ʵ��Ŀ�ģ���A��ӦʢNaOH��Һ����B�з���2Al��2NaOH��2H2O ==== 2NaAlO2��3H2���ķ�Ӧ��
��2��Ŀ���dz�ȥ��þ�Ͻ���������Ĥ��
��3����ȷ˳��ӦΪ�ݢ٢ܢۢڣ�ΪʹC�������ѹǿ��������ѹ��ȣ���ӦʹD��C��Һ����ƽ��
��5����
2Al �� 3H2
2 mol 3 mol
![]() ?
? ![]()
��M��Al��=![]() ��
��
��6����w��Al��=![]() ��100%�ļ��㹫ʽ��֪δϴ�Ӳ���������������������ƫС��
��100%�ļ��㹫ʽ��֪δϴ�Ӳ���������������������ƫС��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�

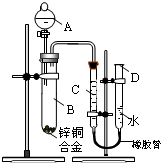 ijѧϰС��������ͼ��ʾװ�òⶨпͭ�Ͻ���п��ͭ������������
ijѧϰС��������ͼ��ʾװ�òⶨпͭ�Ͻ���п��ͭ������������