��Ŀ����
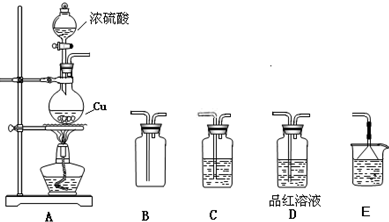
ij�о���ѧϰС��������ͼ��ʾ��װ�ý���Na2O2��H2O��Ӧ��ʵ�顣
��1��������֤���������ɵ�ʵ�鷽����____________��
��2��������q����ˮ�У���Ӧ�����е��ܿ�������ð����˵��_____________________��
��3�����Թ��м�ˮ��������ȫ�ܽ��Ҳ������������ɺ�ȡ���Թܣ����Թ��е���ʵ���ҳ�����ɫAָʾ�����ɹ۲쵽��Һ��죬���ɫ��ȥ�����ָʾ��Ϊ______________��
��4����С��ͬѧ���������Ϻ²��ɫ��ȥ��ԭ���ж���һ��NaOH��Һ��Ũ�ȹ����Ƿ�Ӧ�����п�����H2O2��H2O2����Ư����ָʾ����Ϊ���ų�ǰ�ߵIJ²⣬�ɲ�ȡ�ķ�����____________________��
��5��Ϊ��һ��̽��H2O2�Ƿ��ܹ�����ָʾ����Һ��ijͬѧ��3mL30% H2O2��Һ�е���2��Ʒ��ָʾ�������������Լ3min����Һ��ɫ�����Ըı䡣���������0.5mol/LNaOH��Һ�ɿ�����������С���ݣ�����Һ��ɫ��Ѹ����ȥ�����������______________���������ݵķ�Ӧ�Ļ�ѧ����ʽΪ_________________��
��1���������ǵ�ľ����������p����ľ����ȼ
��2���÷�Ӧ����
��3����̪��Һ
��4�����Թ���ȡ��������Һ��ˮϡ�ͣ��ٵ���Aָʾ�����۲��ɫ�Ƿ���ȥ
��5��H2O2��������������Ư��ָʾ�������ڼ��������¿�Ѹ�ٽ�ָʾ�������� 2H2O2
�����������

��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
�����Ŀ
ij�о���ѧϰС������ͼ��ʾװ���о���ͬ����֮���ת�����⡣
����˵����ȷ���ǣ� ��
| A���Ͽ�����S1�����¿���S2����ʱ���ɵ�װ�����ڵ��� |
| B���Ͽ�����S1�����¿���S2����ѧ��ת��Ϊ���ܣ�����ת��Ϊ���ܵ� |
| C���Ͽ�����S2�����¿���S1����ʱ���ɵ�װ������ԭ��� |
| D���Ͽ�����S2�����¿���S1����ѧ��ת��Ϊ���� |
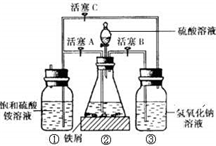 Ħ����[��NH4��2SO4?FeSO4?6H2O]�ڿ����б�һ���������ȶ����ǻ�ѧ�����г��õĻ�ԭ����ij�о���ѧϰС������ͼ��ʾ��ʵ��װ������ȡĦ���Σ�ʵ�鲽�����£��ش��������⣺
Ħ����[��NH4��2SO4?FeSO4?6H2O]�ڿ����б�һ���������ȶ����ǻ�ѧ�����г��õĻ�ԭ����ij�о���ѧϰС������ͼ��ʾ��ʵ��װ������ȡĦ���Σ�ʵ�鲽�����£��ش��������⣺