��Ŀ����
(16��)ֱ������̼-̼���ķ�Ӧ��ʵ�ָ�Ч����ɫ�л��ϳɵ���Ҫ;������������ż����Ӧ�ǽ��걶�ܹ�ע��һ��ֱ������̼-̼���������ͷ�Ӧ�����磺
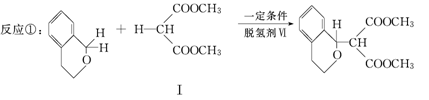
�������������ºϳ�·��ã�
��(����ʽΪC3H8O2)������
(1)�������ķ���ʽΪ ��
����ȫˮ��Ļ�ѧ����ʽΪ (ע������)��
(2)������II�������ᷴӦ�Ļ�ѧ����ʽΪ (ע������)��
(3)�������û�����ԣ���ṹ��ʽΪ �����һ��ͬ���칹������뱥�� NaHCO3��Һ��Ӧ�ų�CO2����������Ľṹ��ʽΪ ��
(4)��Ӧ����1���������(�ṹ��ʽ��ͼ)���ӻ��2����ԭ�Ӻ�ת���1�������廯������ӡ��÷����廯������ӵĽṹ��ʽΪ________��
(5)1 ���� �� 1 ����
�� 1 ����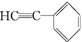 ��һ�������¿ɷ������Ʒ�Ӧ�ٵķ�Ӧ���������ӵĽṹ��ʽΪ________��1 mol �ò���������________mol H2�����ӳɷ�Ӧ��
��һ�������¿ɷ������Ʒ�Ӧ�ٵķ�Ӧ���������ӵĽṹ��ʽΪ________��1 mol �ò���������________mol H2�����ӳɷ�Ӧ��

�������������ºϳ�·��ã�
��(����ʽΪC3H8O2)������
(1)�������ķ���ʽΪ ��
����ȫˮ��Ļ�ѧ����ʽΪ (ע������)��
(2)������II�������ᷴӦ�Ļ�ѧ����ʽΪ (ע������)��
(3)�������û�����ԣ���ṹ��ʽΪ �����һ��ͬ���칹������뱥�� NaHCO3��Һ��Ӧ�ų�CO2����������Ľṹ��ʽΪ ��
(4)��Ӧ����1���������(�ṹ��ʽ��ͼ)���ӻ��2����ԭ�Ӻ�ת���1�������廯������ӡ��÷����廯������ӵĽṹ��ʽΪ________��

(5)1 ����
 �� 1 ����
�� 1 ���� ��һ�������¿ɷ������Ʒ�Ӧ�ٵķ�Ӧ���������ӵĽṹ��ʽΪ________��1 mol �ò���������________mol H2�����ӳɷ�Ӧ��
��һ�������¿ɷ������Ʒ�Ӧ�ٵķ�Ӧ���������ӵĽṹ��ʽΪ________��1 mol �ò���������________mol H2�����ӳɷ�Ӧ����1��C5H8O4

(2)CH2OH-CH2-CH2OH+2HBr CH2Br-CH2-CH2Br+2H2O
CH2Br-CH2-CH2Br+2H2O
(3)OHC-CH2-CHO CH2=CH-COOH
(4)
(5) 8
8

(2)CH2OH-CH2-CH2OH+2HBr
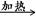 CH2Br-CH2-CH2Br+2H2O
CH2Br-CH2-CH2Br+2H2O(3)OHC-CH2-CHO CH2=CH-COOH
(4)

(5)
 8
8���⿼�����л�������Ľṹ�����ʣ��ѵ��Ƕ����Ѹ���Ϣ��Ǩ��������
��1��������I���ڶ������ɷ���ˮ�ⷴӦ��
��2���ۺϷ����ж�IIΪ��CH2OH-CH2-CH2OH�������������������ᷢ��ȡ����Ӧ���ɶ�±����
��3��II����Ϊ��ԪȩIII��OHC-CH2-CHO����һ��ͬ���칹������뱥�� NaHCO3��Һ��Ӧ�ų�CO2�������С�COOH���ٿ��ǵ������Ͷȿ�֪VΪ��CH2=CH-COOH
��4���������������C=O�Ͽ��ֱ���Hԭ�Ӽӳ�Ϊ��OH����Ԫ��ת��Ϊ������
��5��ǰ�ߡ�CH3�е�C��H�Ͽ������ߡ�C��CH�е�C��H�Ͽ������е����ӵ�����̼ԭ���γ��¼����õ��� �����б�����̼̼���������������ӳɡ�
�����б�����̼̼���������������ӳɡ�
��1��������I���ڶ������ɷ���ˮ�ⷴӦ��
��2���ۺϷ����ж�IIΪ��CH2OH-CH2-CH2OH�������������������ᷢ��ȡ����Ӧ���ɶ�±����
��3��II����Ϊ��ԪȩIII��OHC-CH2-CHO����һ��ͬ���칹������뱥�� NaHCO3��Һ��Ӧ�ų�CO2�������С�COOH���ٿ��ǵ������Ͷȿ�֪VΪ��CH2=CH-COOH
��4���������������C=O�Ͽ��ֱ���Hԭ�Ӽӳ�Ϊ��OH����Ԫ��ת��Ϊ������
��5��ǰ�ߡ�CH3�е�C��H�Ͽ������ߡ�C��CH�е�C��H�Ͽ������е����ӵ�����̼ԭ���γ��¼����õ���
 �����б�����̼̼���������������ӳɡ�
�����б�����̼̼���������������ӳɡ�
��ϰ��ϵ�д�
�����Ŀ
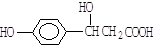 �������Է����ķ�Ӧ������
�������Է����ķ�Ӧ������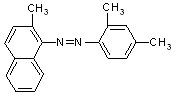
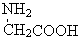 )�ͱ�����(
)�ͱ�����(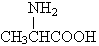 )���������γ�3�ֶ���
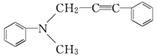
)���������γ�3�ֶ��� N2��+��CH2��̼ϩ��ϩ��������ӳɷ�Ӧ�����ɺ���Ԫ���Ļ����
N2��+��CH2��̼ϩ��ϩ��������ӳɷ�Ӧ�����ɺ���Ԫ���Ļ����
 ��
��