题目内容
【题目】肼(N2H4)是火箭发动机的燃料,它与N2O4反应时,N2O4为氧化剂,生成氮气和水蒸气。已知:①N2(g)+2O2(g)=N2O4(g)ΔH=+8.7kJ·mol-1;②N2H4(g)+O2(g)=N2(g)+2H2O(g)ΔH=-534.0kJ·mol-1;下列表示肼跟N2O4反应的热化学方程式正确的是
A.2N2H4(g)+N2O4(g)=3N2(g)+4H2O(g)ΔH=-542.7kJ·mol-1
B.2N2H4(g)+N2O4(g)=3N2(g)+4H2O(g)ΔH=-1059.3kJ·mol-1
C.N2H4(g)+![]() N2O4(g)=
N2O4(g)=![]() N2(g)+2H2O(g)ΔH=-1076.7kJ·mol-1
N2(g)+2H2O(g)ΔH=-1076.7kJ·mol-1
D.2N2H4(g)+N2O4(g)=3N2(g)+4H2O(g)ΔH=-1076.7kJ·mol-1
【答案】D
【解析】
肼(N2H4)是火箭发动机的燃料,它与N2O4反应生成氮气和水蒸气,已知:①N2(g)+2O2(g)=N2O4(g) ΔH=+8.7kJ·mol-1、②N2H4(g)+O2(g)=N2(g)+2H2O(g) ΔH=-534.0kJ·mol-1,将方程式②×2-①得N2H4跟N2O4反应的热化学方程式,2N2H4(g)+N2O4(g)=3N2(g)+4H2O(g) ΔH=-1076.7kJ·mol-1或N2H4(g)+![]() N2O4(g)=
N2O4(g)=![]() N2(g)+2H2O(g) ΔH=-538.35kJ·mol-1,D符合题意;
N2(g)+2H2O(g) ΔH=-538.35kJ·mol-1,D符合题意;
答案选D。

 期末好成绩系列答案
期末好成绩系列答案 99加1领先期末特训卷系列答案
99加1领先期末特训卷系列答案 百强名校期末冲刺100分系列答案
百强名校期末冲刺100分系列答案【题目】镍电池广泛应用于混合动力汽车系统,电极材料由Ni(OH)2、碳粉、氧化铁等涂覆在铝箔上制成。由于电池使用后电极材料对环境有危害。某兴趣小组对该电池电极材料进行资源回收研究,设计实验流程如下:
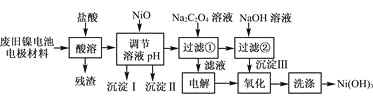
已知:①NiCl2易溶于水,Fe3+不能氧化Ni2+。
②已知实验温度时的溶解度:NiC2O4>NiC2O4·H2O>NiC2O4·2H2O
③某温度下一些金属氢氧化物的Ksp及沉淀析出的理论pH如下表所示:
|
| 开始沉淀pH | 沉淀完全pH |
|
|
|
|
|
|
|
|
|
|
|
|
回答下列问题:
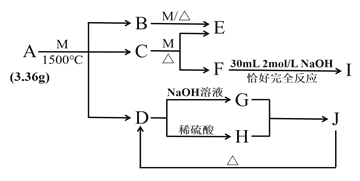
(1) 用NiO调节溶液的pH,依次析出沉淀Ⅰ________和沉淀Ⅱ__________(填化学式)。
(2) 写出加入Na2C2O4溶液的反应的化学方程式:_____________________。
(3) 检验电解滤液时阳极产生的气体的方法:___________________________。
(4) 写出“氧化”反应的离子方程式:___________________________________。
(5) 如何检验Ni(OH)3已洗涤干净?_______________________。