��Ŀ����
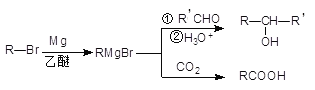
����Ŀ�����������Լ���ơ������Լ��������л�±���������þ����ˮ���ѻ������(THF)�з�Ӧ�Ƶã����л��ϳ�����;�㷺����Ӧԭ�����£�
��A( ![]() )�ϳ� I (
)�ϳ� I ( ![]() )������ͼ��
)������ͼ��
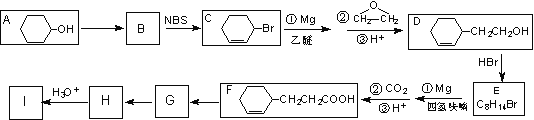
����֪��NBSΪ�廯�Լ�����һ������������ϩ�������������ϩ������
(1) ����A������Ϊ_________________����A����B�ķ�Ӧ������_________��
(2) д����A�Ʊ� ![]() �Ļ�ѧ����ʽ_________________________��
�Ļ�ѧ����ʽ_________________________��
(3) �������һ���������ܼ��������( ![]() )����õ���������Ľṹ��ʽΪ_____________________��
)����õ���������Ľṹ��ʽΪ_____________________��
(4) д����G����H�Ļ�ѧ����ʽ_____________________________________��
(5) д����������Ҫ���I��ͬ���칹��Ľṹ��ʽ_____________________��(д��һ�ּ��ɣ�ͬһ̼�ϲ�������2���ǻ�)
�� �����廯���� �� ��Ԫ�� �� ��������5�ֻ�ѧ������H
(6) ����������Ϣ����A��A�ƵõĻ���ͪ( ![]() )Ϊԭ�Ϻϳ�
)Ϊԭ�Ϻϳ�![]() ��
��![]() ��______________________��
��______________________��
���𰸡� ������ ��ȥ��Ӧ ![]()
![]()
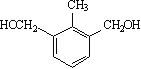 ��
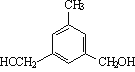
��
![]()
����������1������A(![]() )������Ϊ��������Bת��ΪC���廯��Ӧ������ԭ�ӣ���BΪ
)������Ϊ��������Bת��ΪC���廯��Ӧ������ԭ�ӣ���BΪ![]() ����
����![]() ����
����![]() �ķ�Ӧ��������ȥ��Ӧ����2����A�Ʊ�
�ķ�Ӧ��������ȥ��Ӧ����2����A�Ʊ�![]() �Ǵ���������ͪ����Ӧ�Ļ�ѧ����ʽΪ��
�Ǵ���������ͪ����Ӧ�Ļ�ѧ����ʽΪ��![]() ����3���������һ���������ܼ�������� (
����3���������һ���������ܼ�������� (![]() )����õ�����������в�����̼̼˫������ṹ��ʽΪ��
)����õ�����������в�����̼̼˫������ṹ��ʽΪ��![]() ����4��I (
����4��I ( ![]() )��H�ữ���ã���HΪ
)��H�ữ���ã���HΪ![]() �����F��H���Ƴ�GΪ
�����F��H���Ƴ�GΪ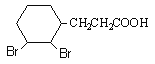 ��
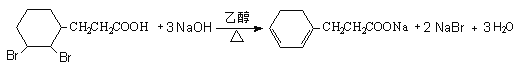
�� ��ǿ��Ĵ���Һ�з�����ȥ��Ӧ����H��
��ǿ��Ĵ���Һ�з�����ȥ��Ӧ����H��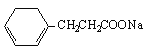 ���Ļ�ѧ����ʽΪ��
���Ļ�ѧ����ʽΪ�� ����5��
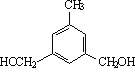
����5��![]() ��ͬ���칹�壬�����������廯������б���������Ԫ�������ж������ǻ�������������5�ֻ�ѧ������H����߶ȶԳƣ����������Ľṹ��ʽ�У�
��ͬ���칹�壬�����������廯������б���������Ԫ�������ж������ǻ�������������5�ֻ�ѧ������H����߶ȶԳƣ����������Ľṹ��ʽ�У� 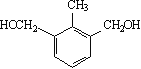 ��
�� ����6������������Ϣ�� A��
����6������������Ϣ�� A��![]() �����廯�ⷢ��ȡ����Ӧ����
�����廯�ⷢ��ȡ����Ӧ����![]() ��
��![]() �ڸ����Լ��Լ��뻷��ͪ(
�ڸ����Լ��Լ��뻷��ͪ(![]() )��ת��Ϊ
)��ת��Ϊ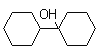 ��
�� ��Ũ������·�����ȥ��Ӧ�Ƶ�
��Ũ������·�����ȥ��Ӧ�Ƶ�![]() ���ϳ�·��Ϊ��
���ϳ�·��Ϊ��![]() ��
��

 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д�