��Ŀ����
�������й�ʵ��������У���������
����������ƽ��ȡ25.20 gNaCl����
���������������������ƹ���
��ʹ������ƿ�ĵ�һ���������Ƚ�����ƿ������ˮϴ�Ӻ���
����25 mL��ʽ�ζ�����ȡ14.80 mL 1 mol/L NaOH��Һ
����ʪ���pH��ֽ�ⶨij��Һ��pH
��ʵ���������Ȼ�����Һʱ�������Ƚ��Ȼ����ܽ��������У������Ƶ�����Ҫ��Ũ��
����������ƽ��ȡ25.20 gNaCl����
���������������������ƹ���
��ʹ������ƿ�ĵ�һ���������Ƚ�����ƿ������ˮϴ�Ӻ���
����25 mL��ʽ�ζ�����ȡ14.80 mL 1 mol/L NaOH��Һ
����ʪ���pH��ֽ�ⶨij��Һ��pH
��ʵ���������Ȼ�����Һʱ�������Ƚ��Ȼ����ܽ��������У������Ƶ�����Ҫ��Ũ��
| A���ڢܢ� | B���ڢۢ� | C���٢ڢ� | D���ڢۢܢݢ� |
A
���������������ƽֻ�ܶ�����0.1g���ٲ���ȷ�����������������Ʋ���Ӧ�����������������������ƹ��壬����ȷ��ʹ������ƿ�ĵ�һ�������Ǽ�©���۲���ȷ������������ǿ�Ӧ���ü�ʽ�ζ�����ȡ������ȷ����pH��ֽ�ⶨij��Һ��pHʱ����ֽ��������ʪ�ݲ���ȷ���Ȼ�������ˮ��������ˮ�⣬��Һ�����ԣ�����ʵ���������Ȼ�����Һʱ�������Ƚ��Ȼ����ܽ��������У�������������ˮ�⣬Ȼ�������Ƶ�����Ҫ��Ũ�ȣ�����ȷ����ѡA��
��������ѧʵ�鳣��������ʹ�÷����ͻ�ѧʵ����������ǽ��л�ѧʵ��Ļ������Ի�ѧʵ��Ŀ����벻����ѧʵ��Ļ������������Թ���������Ҫ���Գ���������ѡ�á�ʵ���������Ϊ���ģ�ͨ����ʲô��Ϊʲô���������ص㿼��ʵ����������Ĺ淶�Ժ�ȷ�Լ��������֪ʶ���ʵ�������������

��ϰ��ϵ�д�
�����Ŀ



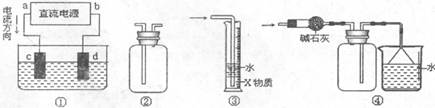
 ��NH
��NH ��C1
��C1