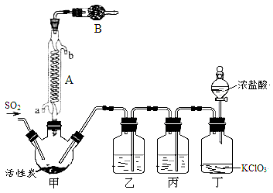
��Ŀ����
����Ŀ������Ĵ��ѽ�ɰ��������ַ�ʽ���У�C4H10��C2H6+C2H4�� C4H10��CH4+C3H6��ij��ѧ��ȤС���ͬѧΪ̽�������ѽ�����CH4��C2H6�ı�����ϵ�������ͼ��ʾʵ�飺
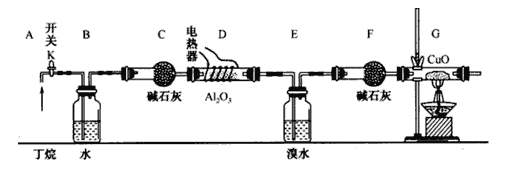
ע��CuO�ܽ���������CO2��H2O��A12O3�Ƕ����ѽ�Ĵ�����G����װ����ʡ�ԡ���ͼ���Ӻ�װ�ú�(���ּг�װ������ȥ)������е�ʵ������У�
�ٸ�D��Gװ�ü��ȣ��ڼ������װ�õ������ԣ����ž�װ���еĿ�����
��1���������������Ⱥ�˳��������____________��
��2����Ҫ˵����������ž��ķ�����_______________________________________________��
��3��Bװ�������������__________________________________________��
��4�����趡����ȫ�ѽ⣬������װ���е���������ȫ��Ӧ����(E��F)װ�õ��������ȷ�Ӧǰ������ 1.82g, Gװ���й������������� 4.l6g��������ѽ������n(CH4)��n(C2H6)=_____________��
��5������Eװ���еĻ�����ٰ���������ʵ�飺

�ٷ������I�����������I_____________�� II_______________��
��Na2SO3��Һ�������ǣ������ӷ���ʽ��ʾ��__________________________________��
���𰸡� �ڢۢ� ��һС�Թ��ռ���Gװ���Ҷ˵��ܿ��ݳ������壬��ȼ�����䴿�ȣ��ޱ����������ž� ͨ���۲����ݣ�������ťA�������������� 3:2 ��Һ ���� SO32-+Br2+H2O=2H++SO42-+2Br-
����������1���漰�����Ʊ������ʵ�ʵ�鶼Ӧ�ȼ��װ�������ԣ���ʵ���漰������ѽ⣬����ڼ���֮ǰ���ž�װ���е�����������D��Gװ�ü��ȣ��ʴ�Ϊ���ڢۢ١�
��2����ͨ������ʵ���������Ĵ��ȣ���ȷ���Ƿ��ž���������һС�Թ��ռ���Gװ���Ҷ˵��ܿ��ݳ������壬��ȼ�����䴿�ȣ��ޱ����������ž���
��3��װ��B�����������ͨ���۲����ݣ������������٣��ʴ�Ϊ��ͨ���۲����ݣ�������ťA����������������
��4�������ѽ����ɵ���ϩ���������ʵ�����ȣ����ɵļ���ͱ�ϩ���ʵ�����ȣ�E��F���յ���ϩ����Gװ�ü��ٵ�����������ͭ����Ԫ�ص���������C2H4�����ʵ���Ϊx��C3H6�����ʵ���Ϊy��������ͼ�������ʵ����ֱ�Ϊx��y��28g��mol-1x+42 g��mol-1y=1.82g�����顢���������ͭ��Ӧ������ԭ�ӵ����ʵ���Ϊ7x+4y=4.16g��16 g��mol-1=0.26mol�����x=0.02mol��y=0.03mol�����Լ�������������ʵ���֮��Ϊ3��2��
��5��E�к����嵥�ʺ���ϩ����ϩ�����嵥�ʵļӳɲ�����мӳɲ������л���������м�������������Һ����ȥ�嵥�ʣ�������ӦΪ��SO32-+Br2+H2O=2H++SO42-+2Br-����Һ�ֲ㣬�ʲ��÷�Һ����������ˮ��Һ���л��㣻���ܵ�Һ�壬���������X��Y��