��Ŀ����
��11�֣�������ijѧϰС����Ҷ����ijЩ���ʽ����о���ѧϰ�Ĺ��̣�
[�о�����]̽���Ҷ����ijЩ����
[��������]�Ҷ��ᣨHOOC��COOH���׳Ʋ��ᣬ����Ҫ�����������£�
��֪��
������100��ʱ��ʼ������157��ʱ��������������ʼ�ֽ⡣
����Ʋ�����ˮ��
����������ʹ����ʯ��ˮ����ǡ�
���������ڵ����¿�����Ϊ���塣
�������������ṩ����Ϣ���ش��������⣺
[�������]
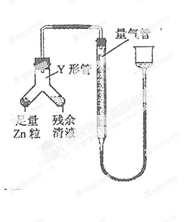
������һ�����ݲ��ᾧ�����ɶ���ֽ������в���
��Ʒ�����
��1����С��ͬѧ���������ΪCO��CO2��H2O����������װ�����һ��̽��ʵ��װ�ã����ᾧ��ֽ�װ���ԣ�װ�ÿ��ظ�ʹ�ã����ӵ�����ȥ����
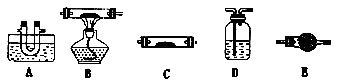
A��ˮ��װ��ˮ����B��װ����ͭ��C��װ��ˮ����ͭ��D��װ����ʯ��ˮ��E��װ��ʯ��
��ش��������⣺
��װ�õ�����˳��Ϊ��A��_____________________________________________��
�ڼ��������CO��ʵ��������____________________________________________________________
������װ���Ƿ���ڲ�����֮���� �����ǻ�����и���ν��___________________________________________________________________________
����������Ҷ������������
��Ʒ�����
��2����С��ͬѧΪ��֤����������������������ʵ�飬�����ܴﵽʵ��Ŀ����______������ĸ����
A�������ᾧ�����ں���̪��NaOH��Һ�У���Һ��ɫ
B���ⶨ��ͬŨ�ȵIJ����������Һ��pH
C���ⶨ�����ƣ�Na2C2O4����Һ��pH
D����������Һ����Na2CO3��Һ�У���CO2�ų�
�����������Ҷ�����л�ԭ��
��Ʒ�����
��3����С��ͬѧ���������ữ��KMnO4��Һ�е�������IJ�����Һ����������KMnO4��Һ��ɫ���Ӷ��жϲ�����н�ǿ�Ļ�ԭ�ԡ���ƽ�÷�Ӧ�����ӷ���ʽ��
___MnO4��+___H2C2O4 +___H+ ===___Mn2+ +___CO2��+___H2O
��4����������ԭ���ɶ����ⶨij���ᾧ����Ʒ������H2C2O4��2H2O������һЩ���ʣ���H2C2O4��2H2O�ĺ�����
�����ǣ���ȡ����Ʒ0.12 g��������ˮ��ȫ�ܽ⣬Ȼ����0.020 mol��L��1
������KMnO4��Һ�ζ����յ㣨���ʲ����뷴Ӧ�����ζ�ǰ��ζ����е�Һ�������ͼ��ʾ����λ��mL������ò��ᾧ����Ʒ��H2C2O4��2H2O����������Ϊ_____________��
����֪���ԭ��������Mr(H2C2O4��2H2O)=126��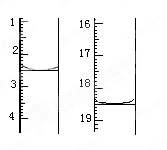
[�о�����]̽���Ҷ����ijЩ����
[��������]�Ҷ��ᣨHOOC��COOH���׳Ʋ��ᣬ����Ҫ�����������£�
| ���� | �Ҷ��� | �Ҷ��ᾧ�� |
| ����ʽ | H2C2O4 | H2C2O4��2H2O |
| ��ɫ״̬ | ��ɫ���� | ��ɫ���� |
| �ܽ�ȣ�g�� | 8.6��20�棩 | �� |
| �۵㣨�棩 | 189.5 | 101.5 |
| �ܶȣ�g��cm��3�� | 1.900 | 1.650 |
������100��ʱ��ʼ������157��ʱ��������������ʼ�ֽ⡣
����Ʋ�����ˮ��
����������ʹ����ʯ��ˮ����ǡ�
���������ڵ����¿�����Ϊ���塣
�������������ṩ����Ϣ���ش��������⣺
[�������]
������һ�����ݲ��ᾧ�����ɶ���ֽ������в���
��Ʒ�����
��1����С��ͬѧ���������ΪCO��CO2��H2O����������װ�����һ��̽��ʵ��װ�ã����ᾧ��ֽ�װ���ԣ�װ�ÿ��ظ�ʹ�ã����ӵ�����ȥ����

A��ˮ��װ��ˮ����B��װ����ͭ��C��װ��ˮ����ͭ��D��װ����ʯ��ˮ��E��װ��ʯ��
��ش��������⣺
��װ�õ�����˳��Ϊ��A��_____________________________________________��
�ڼ��������CO��ʵ��������____________________________________________________________
������װ���Ƿ���ڲ�����֮���� �����ǻ�����и���ν��___________________________________________________________________________
����������Ҷ������������
��Ʒ�����
��2����С��ͬѧΪ��֤����������������������ʵ�飬�����ܴﵽʵ��Ŀ����______������ĸ����
A�������ᾧ�����ں���̪��NaOH��Һ�У���Һ��ɫ
B���ⶨ��ͬŨ�ȵIJ����������Һ��pH
C���ⶨ�����ƣ�Na2C2O4����Һ��pH
D����������Һ����Na2CO3��Һ�У���CO2�ų�
�����������Ҷ�����л�ԭ��
��Ʒ�����
��3����С��ͬѧ���������ữ��KMnO4��Һ�е�������IJ�����Һ����������KMnO4��Һ��ɫ���Ӷ��жϲ�����н�ǿ�Ļ�ԭ�ԡ���ƽ�÷�Ӧ�����ӷ���ʽ��
___MnO4��+___H2C2O4 +___H+ ===___Mn2+ +___CO2��+___H2O
��4����������ԭ���ɶ����ⶨij���ᾧ����Ʒ������H2C2O4��2H2O������һЩ���ʣ���H2C2O4��2H2O�ĺ�����
�����ǣ���ȡ����Ʒ0.12 g��������ˮ��ȫ�ܽ⣬Ȼ����0.020 mol��L��1
������KMnO4��Һ�ζ����յ㣨���ʲ����뷴Ӧ�����ζ�ǰ��ζ����е�Һ�������ͼ��ʾ����λ��mL������ò��ᾧ����Ʒ��H2C2O4��2H2O����������Ϊ_____________��
����֪���ԭ��������Mr(H2C2O4��2H2O)=126��

��11�֣���1����A��C��D��E��B��D��2�֣�
�ں�ɫ�����ɺ�ɫ���ҳ���ʯ��ˮ����ǣ�1�֣�
���ǣ�1�֣� ��β������װ�ã���β��ͨ��ȼ�ŵĵľƾ����ϣ�1�֣�������������Ҳ���֣�
��2��BC��2�֣���ѡ���ۣ�����Ϊֹ��
��3��2,5,6="2,10,8" ��2�֣� ��4��84.0%��2�֣�
�ں�ɫ�����ɺ�ɫ���ҳ���ʯ��ˮ����ǣ�1�֣�
���ǣ�1�֣� ��β������װ�ã���β��ͨ��ȼ�ŵĵľƾ����ϣ�1�֣�������������Ҳ���֣�
��2��BC��2�֣���ѡ���ۣ�����Ϊֹ��
��3��2,5,6="2,10,8" ��2�֣� ��4��84.0%��2�֣�
��1�������ڲ���������ʹ����ʯ��ˮ����ǣ������������ڵ����¿�����Ϊ���壬����Ҫ�ȰѲ���������ȴ��ɹ��塣��ˮ����ͭ��������ˮ�����������ʯ��ˮ��������CO2������ͭ��������CO������ͨ����Һ��Ȼ�����ˮ�������������ȼ���ˮ����������CO����������Ҳ��CO2������Ҫ�ȼ���CO2��������CO��������ͭ��Ӧ��COӦ���Ǹ���ģ�������Ҫ���и��ﴦ���������ȷ��˳��ΪA��C��D��E��B��D
�� CO�ܰ�����ͭ��ԭ���ɺ�ɫ�ĵ���ͭ����CO��Һ������CO2�����Լ��������CO��ʵ�������Ǻ�ɫ�����ɺ�ɫ���ҳ���ʯ��ˮ����ǡ�
������CO���ж����壬������Ҫ����β������װ�ã�����β��ͨ��ȼ�ŵĵľƾ����ϣ�ʹ��ȼ�ա�
��2��A��D��ֻ��˵���Ҷ��������ԣ�������֤�����ڵ���ƽ�⡣����BC����ȷ�ģ�����ĵ�����С����ͬ�����µ�ǿ��ĵ����ԡ������δ���ˮ��ƽ�⣬��ѡBC��
��3������������ԭ��Ӧ�е��ӵĵ�ʧ�غ������ƽ��KMnO4������������Ӧ���̵Ļ��ϼ۴ӣ�7�۽�����2�ۣ��õ�5�����ӡ�H2C2O4�ǻ�ԭ����̼Ԫ�صĻ��ϼ��ɣ�3�ۣ����ߵ���4�ۣ�1mol��ԭ��ʧȥ2mol���ӣ������������ͻ�ԭ�������ʵ���֮����2�U5��������ʽΪ2MnO4��+5H2C2O4 +6H+��2Mn2+ +10CO2��+8H2O��
��4�����ݵζ����еĶ�����֪����������KMnO4��Һ�������18.50ml��2.50mol��16.00ml��������������KMnO4�����ʵ�����0.020mol/L��0.016L��0.00032mol���������Ҷ�����0.00032mol��2��5��0.0008mol����������0.0008mol��126g/mol��0.1008g����������������Ϊ0.1008��0.12��100����84.0��
�� CO�ܰ�����ͭ��ԭ���ɺ�ɫ�ĵ���ͭ����CO��Һ������CO2�����Լ��������CO��ʵ�������Ǻ�ɫ�����ɺ�ɫ���ҳ���ʯ��ˮ����ǡ�
������CO���ж����壬������Ҫ����β������װ�ã�����β��ͨ��ȼ�ŵĵľƾ����ϣ�ʹ��ȼ�ա�
��2��A��D��ֻ��˵���Ҷ��������ԣ�������֤�����ڵ���ƽ�⡣����BC����ȷ�ģ�����ĵ�����С����ͬ�����µ�ǿ��ĵ����ԡ������δ���ˮ��ƽ�⣬��ѡBC��
��3������������ԭ��Ӧ�е��ӵĵ�ʧ�غ������ƽ��KMnO4������������Ӧ���̵Ļ��ϼ۴ӣ�7�۽�����2�ۣ��õ�5�����ӡ�H2C2O4�ǻ�ԭ����̼Ԫ�صĻ��ϼ��ɣ�3�ۣ����ߵ���4�ۣ�1mol��ԭ��ʧȥ2mol���ӣ������������ͻ�ԭ�������ʵ���֮����2�U5��������ʽΪ2MnO4��+5H2C2O4 +6H+��2Mn2+ +10CO2��+8H2O��
��4�����ݵζ����еĶ�����֪����������KMnO4��Һ�������18.50ml��2.50mol��16.00ml��������������KMnO4�����ʵ�����0.020mol/L��0.016L��0.00032mol���������Ҷ�����0.00032mol��2��5��0.0008mol����������0.0008mol��126g/mol��0.1008g����������������Ϊ0.1008��0.12��100����84.0��

��ϰ��ϵ�д�
 �������Ͽ�ʱͬ��ѵ��ϵ�д�
�������Ͽ�ʱͬ��ѵ��ϵ�д� �������ͬ����ϰϵ�д�
�������ͬ����ϰϵ�д�
�����Ŀ
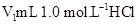 ��Һ��
��Һ��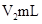 δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ���
δ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ�������¼��Һ�¶ȣ�����ʵ����������ͼ��ʾ(ʵ����ʼ�ձ��� )��
)��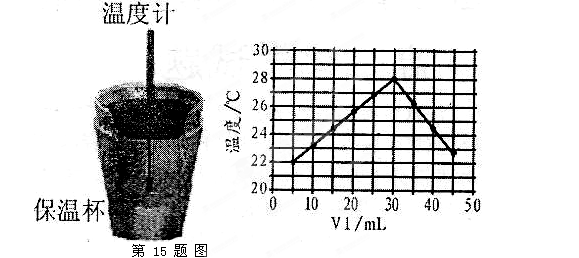 (1)��ʵ��װ���Ͽ�����ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________________��
(1)��ʵ��װ���Ͽ�����ͼ����ȱ�ٵ�һ�ֲ�����Ʒ��________________________��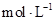
 2CrO42��+2H+���ƣ����µζ��յ��ͺ�
2CrO42��+2H+���ƣ����µζ��յ��ͺ�