��Ŀ����
Ԫ�ػ������֪ʶ����ѧ��ѧ����Ҫ֪ʶ�������й�֪ʶ�ش��������⣺O
��1��NaHCO3�Ƿ��ͷ۵���Ҫ�ɷ�֮һ����Ҫ�������������ֽ�����ʣ���д���÷�Ӧ�Ļ�ѧ����ʽ��__________________________
��2��ʵ���ҵ�Ũ����������ɫƿ���沢�����䰵����ԭ���ǣ��û�ѧ����ʽ˵����
��3������θ����ೣ����������������ҩ���ǣ�
�������ӷ���ʽ˵����
��4��ʵ���������������ӷ���ʽΪ��______________________ �����ڱ�״���£�����11.2����������������HCl�����ʵ���Ϊ mol��
��5��д�����ۺ��������������ȷ�Ӧ�Ļ�ѧ����ʽ �����ȷ�Ӧ�����������ϣ�����ٳ�һ����; ��
��1��2NaHCO3 Na2CO3 + CO2�� + H2O �� 2�֣�
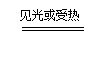
��2��4HNO3 4NO2�� + O2�� + 2H2O �� 2�֣�
��3��Al��OH��3 + 3H+ ==== Al3+ + 3H2O �� 2�֣�

��4��MnO2 + 4 H+ + 2Cl Mn2+ + Cl2�� + 2H2O �� 2�� �� 1 �� 1�֣�

��5��2Al + Fe2O3 2Fe + Al2O3 �� 2�֣�
���Ӹֹ���ƽ����� �� 1�֣�
����:
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�����ڿ�����ȼ�գ����ɵ���ɫ�Ĺ������Ʒ�ĩ | B��þȼ�շ���ҫ�۵İ⣬�����������źŵ������ | C����������¯����Ҫ��CO������ʯ��ԭΪ�� | D������Ӧ�ù㷺�İ뵼����ϣ��������������ά |
| A��þ���ڿ�����ȼ�����ɵ���������MgO�������ڿ�����ȼ�����ɵ���������Na2O | ||||||||
B����2Fe+3Cl2
| ||||||||
| C��CO��NO��NO2���Ǵ�����Ⱦ���壬���ڿ����ж����ȶ����� | ||||||||
| D����CO2ͨ��BaCl2��Һ��������BaCO3����SO2ͨ��BaCl2��Һͬ����������BaSO3 |

