��Ŀ����
(8��) Ԫ�ؼ��仯�����֪ʶ�Ǹ��л�ѧ��Ҫ����ɲ��֣��ǿ��黯ѧ������������ۡ���ѧ���㡢��ѧʵ��֪ʶ�����塣
(1)Ԫ�����ڱ�1��20��Ԫ���У�ij����Ԫ�ص�ԭ���������3�����������1��
������������Ԫ�ص������________�֡�
��������������Ԫ���γɵĻ�������ԭ����֮��Ϊ1��1��
д������һ�ֻ���������ƣ�____________________________��
��2��A����Ȼ�������㷺�Ģ�A��Ԫ�أ����Ի�����F���ڣ��ӵ���A��ʼ������һϵ�л�ѧ��Ӧ������ͼ��ʾ��
��ش��������⣺
��F�Ļ�ѧʽΪ_______ _��D�ĵ���ʽΪ________________
��E��ˮ��Ӧ�Ļ�ѧ����ʽΪ________________________________��
��ʵ�������У�����FΪԭ���Ʊ�����A��д���Ʊ����̵Ļ�ѧ����ʽ�����迼���ۺϾ���Ч�棩
(1) ��7 (2��) ��
�۹������ƻ����(1��)
��2����CaCO3��(1��) 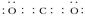 (1��) �� NaH��H2O===NaOH��H2��(1��)
(1��) �� NaH��H2O===NaOH��H2��(1��)
��CaCO3 CaO+CO2 2CaO
CaO+CO2 2CaO 2Ca+O2 (2��)
2Ca+O2 (2��)
��CaCO3+2HCl=CaCl2+CO2+H2O CaCl2 Ca+Cl2(2��)
Ca+Cl2(2��)
�������������(1)��1��20��Ԫ���У�����Ԫ�ص�ԭ���������3�����������1���У�H��Be��He��B,O��Na��F��Mg��Ne��Al��S��K,Cl��Ca��7�顣�γɵĻ�������ԭ����֮��Ϊ1��1����Na2O2����K2S2 ������Ϊ�������ƻ���ء�
��2������Ȼ�������㷺�Ģ�A��Ԫ��ΪCa����CaCO3��ʽ���ڣ�FΪCaCO3����DΪ������̼������ʽ��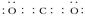 GΪ�����ƣ�CΪ�������ƣ����Na��ˮ�ķ�Ӧ��֪������ˮ��Ӧ��BΪ������EΪNaH����ˮ��Ӧ��NaH��H2O===NaOH��H2����
GΪ�����ƣ�CΪ�������ƣ����Na��ˮ�ķ�Ӧ��֪������ˮ��Ӧ��BΪ������EΪNaH����ˮ��Ӧ��NaH��H2O===NaOH��H2����
��ͨ��Na���Ʊ��ɵã�CaCO3 CaO+CO2 2CaO
CaO+CO2 2CaO 2Ca+O2 (2��)
2Ca+O2 (2��)
��CaCO3+2HCl=CaCl2+CO2+H2O CaCl2 Ca+Cl2
Ca+Cl2
���㣺Ԫ�ؼ��仯����
�����������Ƕ�Ԫ�ؼ��仯����Ŀ��飬���Ǹ�����Ƕ�֪ʶ��Ǩ�ƺ�Ӧ�������Ŀ��顣�ڶ����ʽ����ƶ�ʱ��Ӧ�����ѧ���ʵ����ʡ�

