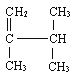
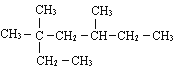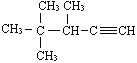��Ŀ����
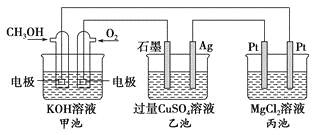
����Ŀ������۲�����װ�ã��ش��������⣺
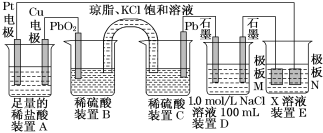
(1)װ��B��PbO2�Ϸ����ĵ缫��Ӧ����ʽΪ________________
(2)װ��A���ܷ�Ӧ�����ӷ���ʽΪ_____________________________��
(3)��װ��E�е�Ŀ������Cu�����϶�������XΪ________������N�IJ���Ϊ________��
(4)��װ��A��Cu�缫�����ı�6.4 gʱ��װ��D�в������������Ϊ________L(��״����)��
(5)��NaOH��Һ���������е�SO2�������õ�Na2SO3��Һ���е�⣬��ѭ������NaOH��ͬʱ�õ�H2SO4����ԭ����ͼ��ʾ(�缫����Ϊʯī)��
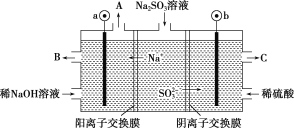
��ͼ��a�����ӵ�Դ��___________��,
��b���缫��ӦʽΪ____________________________________________
���𰸡�PbO2 +2e- +4H++SO42- = PbSO4 +2H2O Cu+2H+![]() Cu2++H2�� AgNO3 Ag 3.92 �� SO32��-2e-+H2O= SO42-+2H+
Cu2++H2�� AgNO3 Ag 3.92 �� SO32��-2e-+H2O= SO42-+2H+
��������
��1��B��Cװ���γ�ԭ��أ�Ǧ��������������Ǧ��������ԭ��طŵ�ʱ�������϶�����Ǧ�õ��Ӻ���������ӷ�Ӧ��������Ǧ��������ԭ��Ӧ��
��2��AΪ���װ�ã�ͭΪ��������������������
��3�����ʱ���Ʋ����������Ƽ����������������Һ�������ӺͶƲ������ͬ��
��4����װ��A��Cu�缫�����ı�6.4gʱ��ת��0.2 mol���ӣ�װ��D��������0. 1mol��n(NaCl)=0. 1mol�������ȷ���2Cl- -2e =Cl2��,��η���4OH --4e -=2H2O+O2������õ�����0.05 mol������0. 025 mol��
��5����Na+������������SO32-����������������a��Ϊ������Ӧ�ӵ�Դ������
��bΪ������SO32-������ʧȥ���ӱ��SO42-��
��1��B��Cװ���γ�ԭ��أ�Ǧ��������������Ǧ��������ԭ��طŵ�ʱ�������϶�����Ǧ�õ��Ӻ���������ӷ�Ӧ��������Ǧ��������ԭ��Ӧ���缫��ӦʽΪ��PbO2 +2e- +4H++SO42- = PbSO4 +2H2O���ʴ�Ϊ��PbO2 +2e- +4H++SO42- = PbSO4 +2H2O��
��2��AΪ���װ�ã�ͭΪ��������������������װ��A���ܷ�Ӧ�����ӷ���ʽΪCu+2H+![]() Cu2++H2�����ʴ�Ϊ��Cu+2H+
Cu2++H2�����ʴ�Ϊ��Cu+2H+![]() Cu2++H2����
Cu2++H2����
��3�����ʱ���Ʋ����������Ƽ����������������Һ�������ӺͶƲ������ͬ����װ��E�е�Ŀ������Cu�����϶�������XΪ���жƲ���������ӵ�AgNO3��Һ������NΪ��������ΪAg���ʴ�Ϊ��AgNO3��Ag��
��4����װ��A��Cu�缫�����ı�6.4gʱ��ת��0.2 mol���ӣ�װ��D��������0. 1mol��n(NaCl)=0. 1mol�������ȷ���2Cl- -2e =Cl2��,��η���4OH --4e -=2H2O+O2������õ�����0.05 mol������0. 025 mol�������Ϊ0.175 mol��22.4Lmol-1=3.92L���ʴ�Ϊ��3.92��
��5����Na+������������SO32-����������������a��Ϊ������Ӧ�ӵ�Դ�������ʴ�Ϊ������
��bΪ������SO32-������ʧȥ���ӱ��SO42-�������缫��ӦʽΪ��SO32��-2e-+H2O= SO42-+2H+���ʴ�Ϊ��SO32��-2e-+H2O= SO42-+2H+��

 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�