��Ŀ����
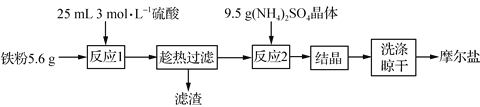
����Ŀ��ʵ�����������Ʊ�Ħ���εIJ����������£�
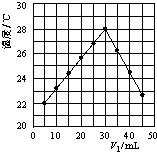
��1�������С����ȡ����˵�ԭ����__________��
��2�� ��Ӧ1�Ƶ��������������ʵ���������ӦС��__________mol��
��3��Ϊȷ��Ħ���εĻ�ѧʽ����������ʵ�飺�ֳ�ȡ4.704 g����Ħ���Σ�����ˮ���Ƴ�100.0 mL��Һ���ٽ�����Һ�ֳ����ȷݡ�
������һ����Һ�м�������Ba(OH)2��Һ���������ݳ���ʹʪ��ĺ�ɫʯ����ֽ
������������ͬʱ���ɰ�ɫ�������漴������Ϊ����ɫ�������к��ɫ��������ϡ���ᴦ������������ַ�Ӧ����ˡ�ϴ�Ӻ������ð�ɫ����2.796 g��
��һ����Һ��0.050 0 mol��L��1 K2Cr2O7������Һ�ζ�����Cr2O![]() ǡ����ȫ����ԭΪCr3��ʱ������K2Cr2O7��Һ�����Ϊ20.00 mL��
ǡ����ȫ����ԭΪCr3��ʱ������K2Cr2O7��Һ�����Ϊ20.00 mL��
��25��ʱ��Ksp(BaSO4)��1.1��10��10����Ҫʹ��Һ��SO![]() ���ӳ�����ȫ(��������Ũ����С��1��10��5 mol��L��1)�����ʱc[(Ba(OH)2]��__________mol��L��1��
���ӳ�����ȫ(��������Ũ����С��1��10��5 mol��L��1)�����ʱc[(Ba(OH)2]��__________mol��L��1��
��ȷ����Ħ���εĻ�ѧʽ(д���������)��___________________
���𰸡� ��ֹ�¶Ƚ��������������������� 0. 075 mol 1. 1��10��5 mol��L��1 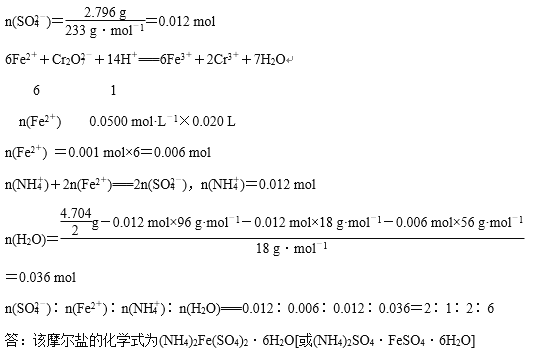
��������Fe��ϡ���ᷴӦ�����������������ȹ��ˣ�Ȼ������Һ�м�������茶��壬����Һ�ᾧ��ϴ�ӡ����ɵõ�Ħ���Ρ�
��1���¶�Խ�ߣ������������ܽ��Խ��Ϊ���������������ܽ�ȣ������С����ȡ����˵�ԭ���ǣ���ֹ�¶Ƚ��ͣ���������������������2��n��Fe��=5.6g/56g��mol��1=0.1mol��n��H2SO4��=3mol��0.025L=0.075mol�����߷�Ӧ����ʽΪFe+H2SO4=H2��+FeSO4�����ݷ���ʽ֪��Feʣ�࣬������Ũ�Ⱥ�ϡʱ�Ͳ���Fe��Ӧ�ˣ����������ϵõ��������������ʵ���С��0.075mol����3����25��ʱ��Ksp(BaSO4)��1.1��10��10����Ҫʹ��Һ��SO![]() ���ӳ�����ȫ(��������Ũ����С��1��10��5 mol��L��1)����c��Ba2����=Ksp(BaSO4)/c(SO42��)=1.1��10-10/10-5mol��L��1=1.1��10��5 mol��L��1���ٸ���Baԭ���غ��c[Ba(OH)2]=c��Ba2����=1.1��10��5 mol��L��1����ȷ����Ħ���εĻ�ѧʽ��Ħ���κ�����������Ӧ�������ᱵ���������������������������������ȶ����������������������ɫ������������ϡ�����ܽ⣬���õ��İ�ɫ������BaSO4��n��BaSO4��=2.796g/233g��mol��1=0.012mol��
���ӳ�����ȫ(��������Ũ����С��1��10��5 mol��L��1)����c��Ba2����=Ksp(BaSO4)/c(SO42��)=1.1��10-10/10-5mol��L��1=1.1��10��5 mol��L��1���ٸ���Baԭ���غ��c[Ba(OH)2]=c��Ba2����=1.1��10��5 mol��L��1����ȷ����Ħ���εĻ�ѧʽ��Ħ���κ�����������Ӧ�������ᱵ���������������������������������ȶ����������������������ɫ������������ϡ�����ܽ⣬���õ��İ�ɫ������BaSO4��n��BaSO4��=2.796g/233g��mol��1=0.012mol��
6Fe2��+Cr2O72��+14H��=6Fe3��+2Cr3��+7H2O
6 1
n 0.0500 mol��L��1��0.020L
n��Fe2����=0.001 mol��6=0.006mol
���ݵ���غ��n��NH4����+2n��Fe2����=2n��SO42������n��NH4����=0.012mol��
n��H2O��=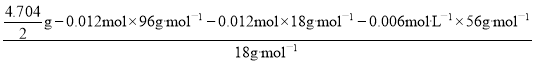 =0.036mol��
=0.036mol��
n��SO42������n��Fe2������n��NH4������n��H2O��=0 012 mol��0 006 mol��0 012mol��0036 mol=2��1��2��6����Ī���εĻ�ѧʽΪ��(NH4)2Fe(SO4)2��6H2O[��NH4��2SO4��FeSO4��6H2O]��